The Hazard Communication Standard Is Found in 29 Cfr
Start Preamble Start Printed Page 9576
AGENCY:
Occupational Safety and Health Administration (OSHA), Labor.
ACTION:
Proposed rule; request for comments.
SUMMARY:
OSHA is proposing through this notice of proposed rulemaking (NPRM) to modify the Hazard Communication Standard (HCS) to conform to the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) Revision 7 (GHS, Rev. 7), to address issues that arose during the implementation of the 2012 update to the HCS, and provide better alignment with other U.S. agencies and international trading partners, without lowering overall protections of the standard. OSHA has preliminarily determined that the proposed revisions to the HCS will reduce costs and burdens while also improving the quality and consistency of information provided to employers and employees regarding chemical hazards and associated protective measures. Consistent with the Executive order entitled "Improving Regulation and Regulatory Review" (January 18, 2011) and section 3(a) of the Regulatory Flexibility Act, which call for assessment and, where appropriate, modification and improvement of existing rules to minimize any significant economic impact upon a substantial number of small entities, OSHA has reviewed the existing HCS. The agency has preliminarily determined that the proposed revisions will enhance the effectiveness of the HCS by ensuring employees are appropriately apprised of the chemical hazards to which they may be exposed, thus reducing the incidence of chemical-related occupational illnesses and injuries. The proposed modifications to the standard include revised criteria for classification of certain health and physical hazards, revised provisions for updating labels, new labeling provisions for small containers, technical amendments related to the contents of safety data sheets (SDSs), and related revisions to definitions of terms used in the standard.
DATES:
Comments on this NPRM (including requests for hearing) and other information must be submitted by April 19, 2021.
Informal public hearing: OSHA will schedule an informal public hearing on the proposed rule if requested during the comment period. If a hearing is requested, the location and date of the hearing, procedures for interested parties to notify the agency of their intention to participate, and procedures for participants to submit their testimony and documentary evidence will be announced in the Federal Register.
ADDRESSES:
Written comments: You may submit comments and attachments, identified by Docket No. OSHA-2019-0001, electronically at http://www.regulations.gov, which is the Federal e-Rulemaking Portal. Follow the instructions online for making electronic submissions. After accessing "all documents and comments" in the docket (Docket No. OSHA-2019-0001), check the "proposed rule" box in the column headed "Document Type," find the document posted on the date of publication of this document, and click the "Comment Now" link. When uploading multiple attachments to regulations.gov, please number all of your attachments because www.regulations.gov will not automatically number the attachments. This will be very useful in identifying all attachments in the preamble. For example, Attachment 1—title of your document, Attachment 2—title of your document, Attachment 3—title of your document. For assistance with commenting and uploading documents, please see the Frequently Asked Questions on regulations.gov.
Instructions: All submissions must include the agency's name and the docket number for this rulemaking (Docket No. OSHA-2019-0001). All comments, including any personal information you provide, are placed in the public docket without change and may be made available online at http://www.regulations.gov. Therefore, OSHA cautions commenters about submitting information they do not want made available to the public, or submitting materials that contain personal information (either about themselves or others), such as Social Security Numbers and birthdates.
Docket: To read or download comments and materials submitted in response to this Federal Register document, go to Docket No. OSHA-2019-0001 at http://www.regulations.gov. All comments and submissions are listed in the http://www.regulations.gov index; however, some information (e.g., copyrighted material) is not publicly available to read or download through that website. All comments and submissions, including copyrighted material, are available for inspection through the OSHA Docket Office.[1]
Start Further Info
FOR FURTHER INFORMATION CONTACT:
For press inquiries: Contact Frank Meilinger, Director, Office of Communications, Occupational Safety and Health Administration, U.S. Department of Labor; telephone: (202) 693-1999; email: meilinger.francis2@dol.gov.
For general information and technical inquiries: Contact Maureen Ruskin, Acting Director, Directorate of Standards and Guidance, Occupational Safety and Health Administration, U.S. Department of Labor; telephone (202) 693-1950 or fax (202) 693-1678; email: ruskin.maureen@dol.gov.
End Further Info End Preamble
SUPPLEMENTARY INFORMATION:
Table of Contents
I. Executive Summary
II. Introduction
III. Events Leading to the Proposed Modifications to the Hazard Communication Standard
IV. Need and Support for the Proposed Modifications to the Hazard Communication Standard
V. Pertinent Legal Authority
VI. OMB Review Under the Paperwork Reduction Act of 1995
VII. Preliminary Economic Analysis and Initial Regulatory Flexibility Analysis
VIII. Federalism
IX. State-Plan States
X. Unfunded Mandates Reform Act
XI. Protecting Children From Environmental Health and Safety Risks
XII. Environmental Impacts
XIII. Consultation and Coordination With Indian Tribal Governments
XIV. Issues and Options Considered
XV. Summary and Explanation of the Proposed Modifications to the Hazard Communication Standard
XVI. Authority and Signature
I. Executive Summary
The Globally Harmonized System of Classification and Labeling of Chemicals (GHS) has been implemented around the world. In 2012, OSHA updated its Hazard Communication Standard (HCS), 29 CFR 1910.1200, to align with Revision 3 of the GHS (77 FR 17574). Start Printed Page 9577 However, the GHS is updated with improvements and clarifications every two years. This proposed rulemaking would amend the HCS to align with Revision 7 of the GHS, published in 2017. OSHA is also proposing updates to address specific issues that have arisen since the 2012 rulemaking and to provide better alignment with international trading partners, without lowering the protections provided by the standard. This action is consistent with Executive Order 13563, "Improving Regulation and Regulatory Review" (January 18, 2011), and the Regulatory Flexibility Act, 5 U.S.C. 610, which requires periodic review of rules that may be out-of-date, ineffective, or excessively burdensome.
OSHA is required by the Occupational Safety and Health Act of 1970 (OSH Act) to assure, as far as possible, safe and healthful working conditions for the Nation's working men and women. As part of this effort, OSHA first promulgated the HCS in 1983 to provide a standardized approach to workplace hazard communications associated with exposure to hazardous chemicals. The HCS requires chemical manufacturers or importers to classify the hazards of chemicals they produce or import. The standard requires all employers to provide information to their employees about the hazardous chemicals to which they are exposed, by means of a hazard communication program, labels and other forms of warning, safety data sheets (SDSs), and information and training. OSHA is not proposing to change the fundamental structure of the HCS.
OSHA has preliminarily determined that the proposed amendments to the HCS would enhance the effectiveness of the standard by ensuring that employees are appropriately apprised of the chemical hazards to which they may be exposed. The proposed modifications to the standard include revised criteria for classification of certain health and physical hazards to better capture and communicate the hazards to downstream users, revised provisions for labels (including proposed provisions addressing the labeling of small containers and the relabeling of chemicals that have been released for shipment), technical amendments related to the contents of SDSs, and new provisions relating to concentrations or concentration ranges being claimed as trade secrets.
Additionally, in accordance with all applicable Executive Orders, the Regulatory Flexibility Act, and the Unfunded Mandates Reform Act, OSHA has prepared a Preliminary Economic Analysis (PEA), including a Preliminary Regulatory Flexibility Analysis Certification, for the proposed modifications to the HCS (see the full PEA in Section VII of this document). Supporting materials prepared by OSHA, such as spreadsheets, are available in the public docket for this rulemaking, Docket ID OSHA-2019-0001, through www.regulations.gov. OSHA invites comments on all aspects of the PEA.
In the PEA, OSHA estimates that the proposed rule would result in net cost savings of $26.8 million per year at a 7 percent discount rate, as shown in Table ES-1, below (a summary of annualized costs by affected industry). Annualized at a 3 percent discount rate, OSHA estimates that the proposed rule would result in net cost savings of $27.5 million per year. Under a perpetual time horizon to allow for cost comparisons under Executive Order 13771, OSHA estimates that the net cost savings of the proposed rule at a discount rate of 7 percent would be $19.6 million per year in 2016 dollars.[2] OSHA also expects that the proposed revisions to the HCS would result in modest improvements in worker health and safety above those already being achieved under the current HCS, but the agency was unable to quantify the magnitude of these health and safety benefits (see Section VII.D. Health and Safety Benefits and Unquantified Positive Economic Effects).
Start Printed Page 9578

Start Printed Page 9579
II. Introduction
This preamble to the proposal to modify the HCS includes a review of the events leading to the proposal, a discussion of the reasons why OSHA believes these modifications are necessary, the preliminary economic and regulatory flexibility analysis for the proposal, and an explanation of the specific revisions OSHA is proposing to make to the standard.
III. Events Leading to the Proposed Modifications to the Hazard Communication Standard
OSHA first promulgated the HCS in 1983, covering only the chemical manufacturing industry (48 FR 53280). The purpose of the standard was to provide a standardized approach for communicating workplace hazards associated with exposure to hazardous chemicals. OSHA updated the HCS in 1987 to expand coverage to all industries where workers are exposed to hazardous chemicals (52 FR 31852). In 1994, OSHA promulgated an additional update to the HCS with technical changes and amendments designed to ensure better comprehension and greater compliance with the standard (59 FR 6126). In adopting the original HCS in 1983, the agency noted the benefits of an internationally harmonized chemical hazard communication standard (48 FR 53287), and actively participated in efforts to develop one over the subsequent decades. In 2012, the agency officially harmonized the HCS with the third revision of the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) (UN GHS, Rev. 3, 2009, Document ID 0085) (77 FR 17574).
OSHA has always envisioned that the HCS would require periodic rulemakings to maintain consistency with the GHS and incorporate the progression of scientific principles and best approaches for classification and communication of workplace hazards related to hazardous chemical exposure (77 FR 17574). This section provides information on the events that have occurred since promulgation of the 2012 HCS, with additional information on the development of the GHS and its relationship to the HCS, and explains the impetus for this proposed rule.
Several international and domestic activities have impacted the direction of the HCS and led to the updates proposed in this NPRM, including negotiations at the UN, OSHA's participation in the U.S.-Canada Regulatory Cooperation Council (RCC) with Health Canada, and information OSHA has received from HCS stakeholders. These are discussed below.
A. International Events Affecting the Standard
The evolution of what was to become the GHS had its early beginnings with the work started in 1956 by the United Nations Economic and Social Council Committee of Experts on the Transport of Dangerous Goods (TDG) and continued in the 1990s through the United Nations Conference on Environment and Economic Development (UNCED), the United Nations International Labour Organization (ILO), and the Organization for Economic Cooperation and Development (OECD) (UN GHS, 2019, Document ID 0053). The overarching goal was to provide an internationally harmonized system to convey information to workers, consumers, and the general public on the physical, health, and environmental effects of hazardous chemicals across the globe, as well as to provide a foundation for the safe management of those chemicals.
Finalized by the UN in 2002, the GHS is intended to harmonize elements of hazard communication, including SDSs and labels, by providing a unified classification system of chemicals based on their physical and health-related hazards. The GHS is updated and revised every two years based on information and experience gained by regulatory agencies, industry, and non-governmental organizations (UN GHS, 2020, Document ID 0052). OSHA largely adopted the third revision to the GHS in 2012.
OSHA leads the U.S. Interagency GHS Coordinating Group, an interagency group that serves as a U.S. delegation to the UN. The Interagency Group works to ensure that modifications to the GHS continue to reflect U.S. agencies' key priorities and do not conflict with U.S. hazard communication and associated requirements. The group meets regularly to discuss issues related to the domestic implementation of the GHS, as well as international work being done at the United Nations Sub-Committee of Experts on the GHS (UNSCEGHS). The Interagency Group consists of representatives from OSHA, the Department of State, the Department of Transportation (DOT), the Environmental Protection Agency (EPA), the U.S. Coast Guard, the Consumer Product Safety Commission (CPSC), the Department of Energy (DOE), the Department of Defense (DOD), and the Bureau of Alcohol, Tobacco, Firearms and Explosives (ATF). To date, OSHA is the only U.S. agency to have implemented the GHS, although CPSC regulations contain elements of the GHS (e.g., precautionary statements) (CPSC, 2006, Document ID 0175). The EPA (which initiated the U.S. working group) has proposed changes to its regulations governing significant new uses of chemical substances under the Toxic Substances Control Act that would align with the HCS and the GHS as well as OSHA's respiratory protection standard (29 CFR 1910.134) and National Institute for Occupational Safety and Health (NIOSH) respirator certification requirements (81 FR 49598).
Since OSHA's adoption of Revision 3 in 2012, the GHS has been updated five times; the latest revision, Revision 8, was published in July 2019 (UN GHS, Rev. 8, 2019, Document ID 0065). Updates to the GHS in Revision 4 (2011) included changes to hazard categories for chemically unstable gases and non-flammable aerosols and updates to, and clarification of, precautionary statements (UN GHS, 2011, Document ID 0240). Changes in Revision 5 of the GHS (2013) included a new test method for oxidizing solids; miscellaneous provisions intended to further clarify the criteria for some hazard classes (skin corrosion/irritation, severe eye damage/irritation, and aerosols) and to complement the information to be included in the SDS; revised and simplified classification and labeling summary tables; a new codification system for hazard pictograms; and revised precautionary statements (UN GHS, 2013, Document ID 0241).
Revision 6 of the GHS (2015) included a new hazard class for desensitized explosives and a new hazard category for pyrophoric gases; miscellaneous provisions intended to clarify the criteria for some hazard classes (explosives, specific target organ toxicity following single exposure, aspiration hazard, and hazardous to the aquatic environment); additional information to be included in section 9 of the SDS; revised precautionary statements; and a new example in Annex 7 addressing labelling of small packages (UN GHS, 2015, Document ID 0134). Changes in Revision 7 (2017) included revised criteria for categorization of flammable gases within Category 1; miscellaneous amendments intended to clarify the definitions of some health hazard classes; additional guidance regarding the coverage of section 14 of the SDS (which is non-mandatory under the HCS); and a new example in Annex 7 addressing labelling of small packages Start Printed Page 9580 with fold-out labels (UN GHS, 2017, Document ID 0094). Revision 8 (published July 2019) includes a change in classification criteria for aerosols (based on flammable properties, heat of combustion); minor changes to precautionary statements for skin irritation and serious eye damage; new provisions for use of non-animal test methods for the skin irritation/corrosion hazard class; and new precautionary pictograms for "keep out of reach of children" (UN GHS, Rev. 8, 2019, Document ID 0065). OSHA is proposing to revise the HCS to align with the GHS Revision 7; however, the agency has included select provisions from Revision 8 for consideration in this rulemaking. Major U.S. trading partners are also aligning with Revision 7. This is discussed in more detail in the introduction to Issues and Options (see Section XIV) and the introduction to Summary and Explanation (see Section XV).
The GHS model is comprehensive and forward-looking, embracing concepts defined in the principles of aggregate exposure and cumulative risk, which have been developed and/or adopted by agencies such as the U.S. EPA pesticides program and NIOSH (US EPA, 2017, Document ID 0054; Lentz, 2015, Document ID 0071). In brief, aggregate exposure considers the combined exposures of a single chemical from multiple pathways (e.g., oral, dermal, inhalation), while cumulative risk evaluates the potential adverse effects from multiple chemicals or stressors (such as heat and noise). Because of its comprehensive approach, the GHS takes into consideration multiple aspects of the intrinsic hazards of a chemical (e.g., physical, health, and environmental hazards) and makes this information available in a manner that facilitates the assessment of aggregate exposures from a single chemical and identifies factors that may contribute to cumulative risk from multiple chemical exposures. While the HCS requires employers to provide information on SDSs in sections 1-11 and 16 (12-15 are non-mandatory) for workplace settings (29 CFR 1910.1200(g)(2)), many consumer products have SDSs available to the public through the National Library of Medicine (NLM, 2020, http://medlineplus.gov/householdproducts.html, Document ID 0059). Thus, aggregate exposure information is available to the public for many chemicals where occupational, consumer, and environmental exposures are possible, as intended by the GHS.
An additional international activity impacting the HCS is OSHA's participation in the RCC. The RCC was established in 2011 to promote economic growth, job creation, and other benefits through increased regulatory coordination and transparency between the U.S. and Canada (US EOP, 2011, Document ID 0057). In June 2018, U.S.-Canada RCC principles were reaffirmed through a memorandum of understanding between the U.S. Office of Information and Regulatory Affairs (OIRA) within the White House Office of Management and Budget and the Treasury Board of Canada (US-Canada MOU, 2018, Document ID 0199). Since the RCC's inception, OSHA and Health Canada, Canada's corresponding governmental agency, have developed joint guidance products and consulted on respective regulatory activities. In keeping with the RCC's goal of regulatory cooperation, OSHA is proposing several updates to the HCS that will align with Canada's Hazardous Products Regulations (HPR), such as changes to exemptions for labeling small containers and using prescribed concentration ranges when claiming trade secrets (Health Canada, 2015, Document ID 0051).
B. Stakeholder Engagement
Since updating the HCS in 2012, OSHA has engaged stakeholders in various ways in order to keep them apprised of changes to the GHS that may have an impact on future updates to the HCS, as well as to gather information about stakeholders' experience implementing the standard. For example, in November 2016, OSHA convened a meeting (International/Globally Harmonized System (GHS), Docket No. OSHA-2016-0005) to inform the public that OSHA was beginning rulemaking efforts to maintain alignment of the HCS with more recent revisions of the GHS. Meeting attendees discussed topics and issues that OSHA should consider during the rulemaking. In addition, attendees provided suggestions as to the types of publications (such as guidance products) that would be helpful in complying with the standard and the topics they would like OSHA to address in future compliance assistance materials.
OSHA has also engaged stakeholders through Interagency Group public meetings, prior to each UNSCEGHS Session, to discuss the issues and proposals being presented at the UN. During this forum, stakeholders have the opportunity to provide comments or voice concerns regarding the various proposals under discussion. Stakeholders are also able to provide comments on these proposals in writing via OSHA's docket for International/Globally Harmonized System (GHS) (Docket No. OSHA-2016-0005). The Interagency Group considers the comments and information gathered at these public meetings and in the docket when developing the United States' position on issues before the UN.
Additionally, in December 2018, the RCC held a stakeholder forum in Washington, DC. The purpose of the forum was to conduct senior-level discussions to proactively identify and discuss challenges, opportunities, and lessons learned regarding Canada-U.S, regulatory cooperation" (US EOP, 2018, Document ID 0252). OSHA led the session regarding chemicals management and workplace chemicals.
C. OSHA Guidance Products, Letters of Interpretation, and Directives
Since OSHA's publication of the HCS update in 2012, the agency has published guidance documents, issued letters of interpretation (LOI), and implemented an enforcement directive. To see the guidance documents, please go to OSHA's web page at: https://www.osha.gov/dsg/hazcom/guidance.html. OSHA will continue to develop guidance documents to assist employers and employees with their understanding of the HCS and is seeking comments in this NPRM on types of guidance documents that the public may find useful to understand the updated HCS. Any guidance provided will accord with the Department's regulation at 29 CFR part 89, with a primary aim of providing helpful, plain language explanations.
OSHA has issued several letters of interpretation (LOI) in response to questions from the regulated community. These LOI provide clarification on provisions in the 2012 update to the HCS, and how they apply in particular circumstances. Some of the major issues covered in the LOI include the labeling of small containers, the labeling of chemicals released for shipment, and the use of concentration ranges for trade secrets. OSHA's LOI on the HCS may be found at https://www.osha.gov/laws-regs/standardinterpretations/standardnumber/1910/1910.1200%20-%20Index/result. In addition, the agency has published a directive that provides guidance to enforcement compliance officers intended to ensure uniform enforcement of the standard by the OSHA field offices (CPL 02-02-079, OSHA, 2015, Document ID 0007; https://www.osha.gov/OshDoc/Directive_pdf/CPL_02-02-079.pdf). Several of the updates in this proposal would codify specific elements of the enforcement guidance the agency has already Start Printed Page 9581 provided in the LOI and the directive (see Section XV: Summary and Explanation for Regulatory Text, Appendix B and Appendix D).
IV. Need and Support for the Proposed Modifications to the Hazard Communication Standard
The HCS is the cornerstone of OSHA's risk mitigation strategy for controlling hazardous chemicals in the workplace. The importance of hazard communication in general and the HCS specifically have been well established over the past few decades, ever since OSHA first established the HCS in 1983 as a worker's "right to know" standard (OSHA Publication 3021—Workers' Rights, 2017). However, even prior to OSHA's promulgation of the HCS, there was recognition that workers needed to know the hazards encountered in the workplace and the importance of communicating, classifying, and training how to address, those hazards. The foundational goal of the HCS is to identify, understand, and communicate the hazards associated with exposure to chemicals before workers experience chronic exposure to those hazards.
OSHA first established the need for the HCS in the 1983 standard (48 FR 53282-53284) and most recently reiterated the need for the standard in 2012, when OSHA adopted the GHS hazard communication framework (77 FR 17584-17600). The 2012 HCS emphasized the need for improved quality, consistency, and comprehensibility of information provided to workers. The improved information mandated by the current HCS enables employers and workers to further reduce risks associated with chemical hazards by enabling them to identify and determine the hazards and by providing a method to indicate the severity of the relevant hazards. The HCS, as updated in 2012, also mandates information on proper storage and handling and other information on risk mitigation and management. Numerous studies examined in the final rulemaking for the 2012 HCS supported the need for a hazard communication standard that was focused on ensuring the comprehensibility of the conveyed information (77 FR 17584-17585).
OSHA is now proposing additional changes to the HCS that will serve three primary purposes: (1) Maintaining alignment with the GHS and ensuring that the standard reflects the current state of science and knowledge on relevant topics; (2) cooperating with international trading partners and other Federal agencies; and (3) responding to stakeholder experiences implementing current HCS requirements. The proposed changes include clarifying the purpose and scope of the standard, adding definitions, codifying enforcement policies currently in OSHA's compliance directive, clarifying requirements related to the transport of hazardous chemicals, adding labeling provisions for small containers, and adopting new requirements related to preparation of SDSs and new provisions related to claiming concentration ranges as trade secrets. The agency believes that the changes proposed in this NPRM will further improve the comprehensibility and utility of the standard and allow the HCS to keep up with advances in relevant science and technology, thereby better protecting worker health and safety.
A. Maintaining Alignment With the GHS and Ensuring That the Standard Reflects the Current State of Science and Knowledge on Relevant Topics
Periodic updates to the HCS are needed to maintain pace with the general advancement of science, technology, and our understanding of the processes involved in effective communication. As stated in the 2008 ILO report, "Continuous improvement of occupational safety and health must be promoted. This is necessary to ensure that national laws, regulations, and technical standards to prevent occupational injuries, disease, and deaths are adapted periodically to social, technical, and scientific progress and other changes in the world of work." (ILO, 2008, Document ID 0181). While the tools and protective measures in place to reduce or prevent chemical-related occupational injuries and illnesses are effective, such tools and systems become less effective as time goes by and new technologies and workplace hazards emerge. Therefore, there is a need for continual improvement in the systems and processes designed to identify, communicate about, and reduce workplace exposures to chemical hazards. OSHA has always intended for the HCS to be updated periodically to reflect these advancements, as is the GHS (for further discussion see Section XIV, Issues and Options).[3]
The proposed changes to the HCS will result in better alignment between the standard and the continually-evolving GHS. The first edition of the UN GHS, adopted in December 2002 and published in 2003, implemented the 16-section format for SDSs that is now standard across much of the globe. As information has improved, the GHS has updated the form and content of SDSs[4] to improve readability, minimize redundancies, and ensure hazards are communicated appropriately (UN GHS, 2017, Document ID 0060; ANS revises standard, 2005, Document ID 0237).
Information OSHA has collected since publication of the 2012 updates to the HCS indicates that aligning the HCS with the GHS has had a positive impact. Data from published studies indicate that the hazard communication approach taken in the 2012 HCS has been effective, when implemented appropriately, in enabling workers to understand, avoid, and mitigate exposures to hazardous chemicals in the workplace (Bechtoldt, 2014, Document ID 0061; Elliott, 2016, Document ID 0119). Industry representatives have indicated that workers responded positively to training on pictograms and hazard statements because it provided an opportunity to address distinctions between acute toxicity and chronic health effects (Bechtold, 2014, Document ID 0061). In reference to SDSs, one industry representative stated that "[b]ecause the standardized hazard statements and classifications are so precisely disclosed, it'll be a lot easier for industrial hygienists to identify the more hazardous chemicals, decide where they may need to take action, and compare the hazards of one product versus another." (Bechtold, 2014, Start Printed Page 9582 Document ID 0061; Elliot, 2016, Document ID 0119). Consistent labeling requirements have also enabled employers to identify the most hazardous materials in the workplace, understand more about the health effects of these chemicals, and address which hazardous chemicals they may want to replace with safer alternatives (Bechtold, 2014, Document ID 0061).
Several studies published since the 2012 HCS adopted the 16-section SDS format indicate that the new format improves comprehension in the workplace (Elliott, 2016, Document ID 0119; Boelhouwer, 2013, Document ID 0107). However, other recent studies have shown that the system can still be improved upon. Multiple studies in various industries have demonstrated that while comprehension has improved, many SDSs lack information vital to worker protections. Problems include insufficient information on the identification of substances/mixtures; inadequate hazard identification and classification information (e.g., missing information on carcinogens and sensitizers, incorrect chemical classifications); lack of precautionary statements on safe handling; missing information on exposure controls/personal protective equipment; and missing toxicological information (Jang, 2019, Document ID 0110; Allen, 2017, Document ID 0117; DiMare, 2017, Document ID 0118; Tsai, 2016, Document ID 0116; Friis, 2015, Document ID 0120; Saito, 2015, Document ID 0191; Suleiman, 2014, Document ID 0192; Lee, 2012, Document ID 0070). A 2014 study concluded that the contents of the SDSs evaluated were generic and incomplete, lacking important safety measures and health information (Suleiman, 2014, Document ID 0192). A study on mixtures found that information on individual ingredients within mixtures was sometimes completely missing and that information on hazard characterization and classification was ambiguous and almost entirely incorrect (LeBouf, 2019, Document ID 0183). Furthermore, a 2012 study conducted by NIOSH found that SDSs for certain classes of chemicals lacked sufficient information to communicate the appropriate hazards and remedies related to engineered nanomaterials (Eastlake, 2012, Document ID 0063). A follow-up NIOSH study found some improvement in SDS preparation since implementation of the 2012 HCS; however, the study also found that there are still serious deficiencies in providing adequate information on the inherent health and safety hazards of engineered nanomaterials, including handling and storage (Hodson, 2019, Document ID 0067).
Inadequate information on the chemical hazards and risk management practices required on SDSs can lead to overexposure to chemical hazards and puts workers at risk. The studies described above demonstrate the need for ongoing review and refinement to make certain the standard is addressing comprehensibility issues and staying relevant with current occupational safety and health tools, science, and technology. Using information gained through the experience of global stakeholders, the GHS is updated with revisions and improvements every two years. These changes have been outlined in brief in Section III (Events Leading to the Proposed Modifications to the Hazard Communication Standard) of this NPRM. The proposed updates to appendix D, which are based in part on recent revisions to the GHS, seek, among other things, to remedy the issues that have been identified by clarifying the information needed in the SDS. For example, the change in section 9 (physical characteristics to include particle characteristics) will identify exposure issues that are not addressed by the current format. This should, among other things, improve the hazard information required for nanomaterials.
Furthermore, the GHS has been updated to reflect the development of non-animal test methods for use in hazard determination and classification. The development of these test methods led to updates in Chapter 3.2 on skin corrosion/irritation that incorporated new in vitro test methods, and computational and in silico techniques, to classify chemicals for this category of hazard (UN GHS, 2018, Document ID 0242). And techniques and processes developed in the behavioral sciences have led to the development of more effective communication practices for occupational safety and health purposes (NIOSH, 2019, Document ID 0126).[5] Studies evaluating the effectiveness of precautionary statements and pictograms used in the GHS have led to their evolution and continued revisions (Fagotto, 2003, Document ID 0125; Ta, 2010, Document ID 0115; Ta, 2011, Document ID 0194; Chan, 2017, Document ID 0017).
In addition to directly enhancing worker protections through improved hazard communication, updating the HCS (based on the GHS) will also improve the availability of important information to support larger efforts to address workplace hazards. For example, NIOSH is exploring the use of aggregate exposures (exposures to a specific chemical or hazard from several different sources) and cumulative risk models for use in setting occupational exposure limits and assessing impacts on worker health (Lentz, 2015, Document ID 0071; Redingert, 2015, Document ID 0100). A real-world example of the potential effects of aggregate exposure comes from the increased use of nanosilver in consumer products. A recent NIOSH review of nanosilver indicates that the current OSHA PEL for silver is adequate to protect workers from silver's adverse health effects (NIOSH, 2018, Document ID 0188). However, a 2013 study looking at the increased presence of nanosilver in consumer products (e.g., use of nanosilver as an antimicrobial in clothing and materials that come into contact with food), and the increased environmental exposures from the manufacture, use, and disposal of these consumer products, indicates that the OSHA PEL may be inadequate to protect workers if nanosilver continues to be added to new consumer products (Balcher, 2013, Document ID 0097). This example highlights the importance of an effective overarching hazard communication strategy in understanding and managing exposures and risk.
Regularly updating the HCS to align with international practices also eases compliance for regulated entities because it provides greater international consistency (Bechtold, 2014, Document ID 0061). Industry groups, such as the American Petroleum Institute (API), have indicated their support for regular HCS updates as long as there is sufficient input from stakeholders (API, 2009, Document ID 0167). During the 2012 rulemaking, numerous safety organizations (including NIOSH, the American Chemical Society (ACS), the American Industrial Hygiene Association (AIHA), the American Society of Safety Engineers (ASSE), and the Society for Chemical Hazard Communication (SCHC)) have publicly supported OSHA's continued updates to the HCS (see 77 FR 17585, 17603, 17604). The Society of Toxicology has also expressed support for updating the HCS to align with the GHS as this "is ani important step toward creating consistent communication about the hazards of chemicals used around the world." (see 77 FR 17585). Start Printed Page 9583
B. Cooperating With International Trading Partners and Other Federal Agencies
In support of the second goal of this NPRM, OSHA expects that the proposed updates to the HCS will facilitate cooperation with international trading partners and other Federal agencies. With respect to the U.S. and Canada specifically, the two countries participate in the RCC, which has a goal to "reduce, eliminate, or prevent unnecessary regulatory differences between both countries while maintaining high levels of protection for health, safety, and the environment" (US-Canada MOU, 2018, Document ID 0252). OSHA continues to work with Health Canada through the RCC to develop guidance documents pertaining to hazard communication issues the two countries share and to work cooperatively through the UN GHS subcommittee (see Section III, Events Leading to the Proposed Modifications to the Hazard Communication Standard). In addition, OSHA and Health Canada share regular updates on regulatory activity. As explained in the Summary and Explanation (see Section XV), a number of the updates OSHA is proposing in this NPRM would align U.S. and Canadian hazard communication practices, thereby facilitating cooperation between the two countries, easing compliance for employers who participate in both markets, and strengthening worker protections by providing harmonized hazard communication standards across trade borders.
In addition, OSHA is proposing to update the requirements for bulk shipment under paragraph (f)(5), Transportation to provide additional clarity for shipments that are also regulated by the U.S. Department of Transportation (DOT). For bulk shipments, the proposed new paragraph would increase flexibility by allowing labels to be placed on the immediate container or transmitted with shipping papers, bills of lading, or by other technological or electronic means so that they are immediately available to workers in printed form on the receiving end of the shipment. And in another effort to facilitate inter-agency cooperation, OSHA is proposing new language for paragraph (f)(5) providing that where a pictogram required by the DOT appears on the label for a shipped container, the HCS pictogram for the same hazard may also be provided, but is not required.
C. Responding to Stakeholder Experiences Implementing the 2012 HCS
Finally, some of the proposed changes in this NPRM, those related to labeling of small containers and relabeling requirements for chemicals that have been released for shipment, were developed in response to feedback and comments received from stakeholders since the promulgation of the 2012 updates to the HCS (Collatz, 2015, Document ID 0174; Ghosh, 2015, Document ID 0180). With respect to the labeling of small containers, issues raised by stakeholders included concerns about insufficient space on the label to highlight the most relevant safety information, problems with the readability of information on small labels, and challenges associated with using fold-out labels for certain small containers that need special handling (Watters, 2013, Document ID 0200; Collaltz, 2015, Document ID 0174; Blankfield, 2017, Document ID 0170). The proposed updates to the HCS related to the labeling of small containers are designed to address these issues. Furthermore, OSHA believes that adopting a uniform standard for the labeling of small containers will enhance worker protections by providing more clarity and certainty about the hazards posed by the chemicals contained in such containers (see Section X Summary and Explanation for (f)(12), Small container labelling).
Similarly, the proposed revisions to paragraph (f)(11), which address the relabeling of chemicals that have been released for shipment, are designed to address stakeholder concerns about the difficulty some manufacturers have in complying with paragraph (f)(11), especially in the case of chemicals that travel through long distribution cycles (Kenyon, 2017, Document ID 0182). Many products have straightforward supply chains and are packaged, labeled, and promptly shipped downstream. Other products, for example in the agrochemical sector, are packaged and labeled when they leave the chemical manufacturer's facility, but may reside at a warehouse or distribution facility for extended periods of time (e.g., several years) before being shipped downstream. There are also instances where products may be returned from the downstream users to the distribution facility and then shipped to other customers (NGFA, 2016, Document ID OSHA-2016-0005-0018; AFIA, 2016, Document ID OSHA-2016-0005-0017). OSHA believes the proposed revisions to paragraph (f)(11) to provide that relabeling is not required for chemicals that have been released for shipment and are awaiting future distribution will accommodate these concerns; the proposal would also maintain worker protections by requiring the chemical manufacturer or importer to provide an updated label for each individual container with each shipment.
V. Pertinent Legal Authority
A. Background
The purpose of the Occupational Safety and Health Act of 1970 (the "OSH Act" or "Act") (29 U.S.C. 651 et seq.) is "to assure so far as possible every working man and woman in the Nation safe and healthful working conditions and to preserve our human resources." 29 U.S.C. 651(b). To achieve this goal, Congress authorized the Secretary of Labor to promulgate occupational safety and health standards pursuant to notice and comment. 29 U.S.C. 655(b). An occupational safety and health standard is a standard "which requires conditions, or the adoption or use of one or more practices, means, methods, operations, or processes, reasonably necessary or appropriate to provide safe or healthful employment and places of employment." 29 U.S.C. 652(8).
The OSH Act also authorizes the Secretary to "modify" or "revoke" any occupational safety or health standard, 29 U.S.C. 655(b), and under the Administrative Procedure Act, regulatory agencies generally may revise their rules if the changes are supported by a reasoned analysis. See Encino Motorcars, LLC v. Navarro, U.S., 136 S. Ct. 2117, 2125-26 (2016); Motor Vehicle Mfrs. Ass'n v. State Farm Mut. Auto. Ins. Co., 463 U.S. 29, 42 (1983). In passing the OSH Act, Congress recognized that OSHA should revise and replace its standards as "new knowledge and techniques are developed." S. Rep. 91-1282 at 6 (1970). The Supreme Court has observed that administrative agencies "do not establish rules of conduct to last forever, and . . . must be given ample latitude to adapt their rules and policies to the demands of changing circumstances." Motor Vehicle Mfrs. Ass'n, 463 U.S. at 42 (internal quotation marks and citations omitted).
Before the Secretary can promulgate any permanent health or safety standard, he must make a threshold finding that significant risk is present and that such risk can be eliminated or lessened by a change in practices. Indus. Union Dep't v. Am. Petroleum Inst., 448 U.S. 607, 642 (1980) (plurality opinion) Start Printed Page 9584 ("Benzene"). As explained more fully below, OSHA need not make additional findings on risk for this proposal because OSHA previously determined that the HCS addresses a significant risk. 77 FR 17603-17604.
In promulgating a standard under, and making the determinations required by, the OSH Act, OSHA's determinations will be deemed conclusive if they are "supported by substantial evidence in the record considered as a whole." 29 U.S.C. 655(f). OSHA must use the "best available evidence," which includes "the latest available scientific data in the field"; "research, demonstrations, experiments, and such other information as may be appropriate"; and "experience gained under this and other health and safety laws." 29 U.S.C. 655(b)(5).
B. Authority—Section 6(b)(5)
The HCS is a health standard promulgated under the authority of section 6(b)(5) of the OSH Act. See Associated Builders & Contractors, Inc. v. Brock, 862 F.2d 63, 67-68 (3d Cir. 1988); United Steelworkers of Am. v. Auchter, 763 F.2d 728, 735 (3d Cir. 1985); 77 FR 17601. Section 6(b)(5) of the OSH Act provides that in promulgating health standards dealing with toxic materials or harmful physical agents, the Secretary must "set the standard which most adequately assures, to the extent feasible, on the basis of the best available evidence, that no employee will suffer material impairment of health or functional capacity even if such employee has regular exposure to the hazard dealt with by such standard for the period of his working life." 29 U.S.C. 655(b)(5). Thus, once OSHA determines that a significant risk due to a health hazard is present and that such risk can be reduced or eliminated by an OSHA standard, section 6(b)(5) requires OSHA to issue the standard, based on the best available evidence, that "most adequately assures" employee protection, subject only to feasibility considerations. As the Supreme Court has explained, in passing section 6(b)(5), Congress "place[d] . . . worker health above all other considerations save those making attainment of this `benefit' unachievable." Am. Textile Mfrs. Inst., Inc. v. Donovan, 452 U.S. 490, 509 (1981) ("Cotton Dust").
C. Other Authority
The HCS is also promulgated under the authority of section 6(b)(7) of the OSH Act. See United Steelworkers, 763 F.2d at 730; 77 FR 17601. Section 6(b)(7) of the OSH Act provides in part: "Any standard promulgated under this subsection shall prescribe the use of labels or other appropriate forms of warning as are necessary to insure that employees are apprised of all hazards to which they are exposed, relevant symptoms and appropriate emergency treatment, and proper conditions and precautions of safe use or exposure." 29 U.S.C. 655(b)(7). Section 6(b)(7)'s labeling and employee warning requirements provide basic protections for employees in the absence of specific permissible exposure limits, particularly by providing employers and employees with information necessary to design work processes that protect employees against exposure to hazardous chemicals in the first instance.
The last sentence of section 6(b)(7) provides that the Secretary, in consultation with the Secretary of Health and Human Services, may by rule promulgated pursuant to section 553 of Title 5, make appropriate modifications in the foregoing requirements relating to the use of labels or other forms of warning, monitoring or measuring, and medical examinations, as may be warranted by experience, information, or medical or technological developments acquired subsequent to the promulgation of the relevant standard. 29 U.S.C. 655(b)(7). OSHA used the authority granted by this paragraph to promulgate the 2012 revisions to the HCS, 77 FR 17602, and this provision provides additional authority for the current proposal.
This proposal to update the HCS fits well within the authority granted by the last sentence of section 6(b)(7). The changes proposed would constitute a "modification" of the HCS regarding "the use of labels or other forms of warning." As explained more fully elsewhere in this preamble, OSHA believes the proposed updates to be "appropriate" based on "experience, information, or medical or technological developments acquired subsequent to the promulgation of the relevant standard." The updates found in GHS Rev. 7 may be considered a "technological development" that has occurred since the promulgation of the HCS in 2012 and are also "warranted by experience [and] information." The GHS was negotiated and drafted through the involvement of labor, industry, and governmental agencies, and thus represents the collective experience and information on hazard communication gathered by the participants in these sectors over the last several decades. See 71 FR 53617, 53618-53619.[6] See also Section III of this preamble, Events Leading to the Proposed Modifications to the Hazard Communication Standard.
Authority for the HCS is also found in section 8, paragraphs (c) and (g), of the OSH Act. Section 8(c)(1) of the OSH Act empowers the Secretary to require employers to make, keep, and preserve records regarding activities related to the OSH Act and to make such records available to the Secretary. 29 U.S.C. 657(c)(1). Section 8(g)(2) of the OSH Act empowers the Secretary to "prescribe such rules and regulations as he may deem necessary to carry out [his] responsibilities" under the Act. 29 U.S.C. 657(g)(2).
D. Significant Risk
As required for standards promulgated under section 6(b)(5) of the OSH Act, OSHA determined that the HCS would substantially reduce a significant risk of material harm. Most OSHA health standards protect employees by imposing requirements when employees are exposed to a concentration of a hazardous substance that OSHA has found creates a significant risk of material health impairment. Thus, in making the significant risk determination in these cases, OSHA measures and assesses the hazards of employee exposures in order to determine the level at which a significant risk arises.
OSHA took a different approach to its significant risk determination when first promulgating the HCS in 1983. Rather than attempting to assess the risk associated with exposures to each hazardous chemical in each industry to determine if that chemical posed a significant risk in that industry, OSHA took a more general approach. It relied on NIOSH data showing that about 25 million or about 25 percent of American employees were potentially exposed to one or more of 8,000 NIOSH-identified chemical hazards and that for the years 1977 and 1978 more than 174,000 illnesses were likely caused by exposure to hazardous chemicals. 48 FR 53282. OSHA then noted the consensus evident in the record among labor, industry, health professionals, and government that an "effective [F]ederal standard requiring employers to identify workplace hazards, communicate hazard information to employees, and train employees in recognizing and avoiding those hazards" was necessary to protect employee health. 48 FR Start Printed Page 9585 53283. OSHA determined that the HCS addressed a significant risk because "inadequate communication about serious chemical hazards endangers workers," and that the practices required by the standard were "necessary or appropriate to the elimination or mitigation of these hazards." 48 FR 53321. The U.S. Court of Appeals for the Third Circuit agreed that "inadequate communication is itself a hazard, which the standard can eliminate or mitigate." United Steelworkers, 763 F.2d at 735. That court has upheld OSHA's determination of significant risk as sufficient to justify the HCS. See Associated Builders & Contractors, 862 F.2d at 67-68 (discussing the history of its review of the issue).
OSHA reaffirmed its finding of significant risk in adopting revisions to the HCS in 1994. See 59 FR 6126-6133. When revising the HCS to adopt the GHS model in 2012, OSHA found that there remained a "significant risk of inadequate communication" of chemical hazards in the workplace and that adopting the standardized requirements of the GHS would substantially reduce that risk by improving chemical hazard communications. 77 FR 17603-17604.
In previous rulemakings, OSHA rejected suggestions that the hazard assessment and communication obligations of the HCS should arise only where the downstream use creates a significant risk because it is difficult, if not impossible, for OSHA or manufacturers and importers to know in advance where these risks might occur. See 48 FR 53295-53296; 59 FR 6132. Further, it is only by the provision of hazard information that downstream employers and employees can determine how to use the chemical so that exposure and risk may be minimized. See 48 FR 53295-53296; 59 FR 6132. Thus, the HCS protects employees from significant risk by requiring communications about all chemicals that may present a hazard to employees, regardless of the exposure or risk levels any particular downstream user might actually experience. See Durez Div. of Occidental Chem. Corp. v. OSHA, 906 F.2d 1, 3-4 (D.C. Cir. 1990); Gen. Carbon Co. v. OSHRC, 860 F.2d 479, 484-85 (D.C. Cir. 1988).
For the changes proposed in this NPRM, OSHA has not made a new preliminary finding of significant risk, but is proposing changes that are reasonably related to the purpose of the HCS as a whole. When, as here, OSHA has previously determined that its standard substantially reduces a significant risk, it is unnecessary for the agency to make additional findings on risk for every provision of that standard. See, e.g., Pub. Citizen Health Research Grp. v. Tyson, 796 F.2d 1479, 1502 n.16 (D.C. Cir. 1986) (rejecting the argument that OSHA must "find that each and every aspect of its standard eliminates a significant risk"). Rather, once OSHA makes a general significant risk finding in support of a standard, the next question is whether a particular requirement is reasonably related to the purpose of the standard as a whole. See Asbestos Info. Ass'n/N. Am. v. Reich, 117 F.3d 891, 894 (5th Cir. 1997); Forging Indus. Ass'n v. Sec'y of Labor, 773 F.2d 1436, 1447 (4th Cir. 1985); United Steelworkers of Am., AFL-CIO-CLC v. Marshall, 647 F.2d 1189, 1237-38 (D.C. Cir. 1980) ("Lead I").
Furthermore, the Supreme Court has recognized that protective measures like those called for by the HCS may be imposed in workplaces where chemical exposure levels are below that for which OSHA has found a significant risk. In Benzene, the Court recognized that the "backstop" provisions of section 6(b)(7) allow OSHA to impose information requirements even before the employee is exposed to the significant risk. See Benzene, 448 U.S. at 657-58 & n.66. Rather than requiring a finding of significant risk, the last sentence of section 6(b)(7) provides other assurances that OSHA is exercising its authority appropriately by requiring the involvement of the Secretary of Health and Human Services, and by limiting the authority only to modifications that are based on "experience, information, or medical or technological developments" acquired since the promulgation of the standard in the limited areas of hazard communication, monitoring, and medical examinations. Therefore, OSHA need not make any new significant risk findings; rather, the final rule is supported by the significant risk findings that OSHA made when it adopted the current HCS.[7] See 77 FR 17602.
E. Feasibility
Because section 6(b)(5) of the OSH Act explicitly requires OSHA to set health standards that eliminate risk "to the extent feasible," OSHA uses feasibility analysis to make standards-setting decisions dealing with toxic materials or harmful physical agents. 29 U.S.C. 655(b)(5); Cotton Dust, 452 U.S. at 509. Feasibility in this context means "capable of being done, executed, or effected." Cotton Dust, 452 U.S. at 508-09. Feasibility has two aspects, economic and technological. Lead I, 647 F.2d at 1264. A standard is technologically feasible if the protective measures it requires already exist, can be brought into existence with available technology, or can be created with technology that can reasonably be expected to be developed. See id. at 1272. A standard is economically feasible if industry can absorb or pass on the cost of compliance without threatening its long-term profitability or competitive structure. See Cotton Dust, 452 U.S. at 530 n.55; Lead I, 647 F.2d at 1265. As discussed more fully in Section VII.E of this preamble, Technological Feasibility, OSHA has preliminarily determined that compliance with the proposed revisions to the HCS is technologically feasible for all affected industries because compliance can be achieved with readily and widely available technologies. As discussed more fully in Section VII.G, Economic Feasibility and Impacts, OSHA has preliminarily determined that the proposed changes to the HCS are economically feasible because employers can comply without threatening the long-term profitability or competitive structure of any affected industries.
VI. OMB Review Under the Paperwork Reduction Act of 1995
A. Overview
OSHA is proposing to revise the Hazard Communication Standard (HCS), 29 CFR 1910.1200, which contains collection of information that are subject to review by the Office of Management and Budget (OMB) under the Paperwork Reduction Act of 1995 (PRA), 44 U.S.C. 3501 et seq., and OMB regulations at 5 CFR part 1320. The agency is planning to revise and update the existing previously-approved paperwork package under OMB control number 1218-0072.
The PRA defines "collection of information" to mean "the obtaining, causing to be obtained, soliciting, or requiring the disclosure to third parties or the public, of facts or opinions by or for an agency, regardless of form or Start Printed Page 9586 format." 44 U.S.C. 3502(3)(A). Under the PRA, a Federal agency cannot conduct or sponsor a collection of information unless OMB approves it and the agency displays a currently valid OMB control number. 44 U.S.C. 3507. Also, notwithstanding any other provision of law, no employer shall be subject to penalty for failing to comply with a collection of information if the collection of information does not display a currently valid OMB control number. 44 U.S.C. 3512.
B. Solicitation of Comments
OSHA prepared and submitted an Information Collection Request (ICR) to OMB proposing to revise certain collection of information currently contained in that paperwork package in accordance with 44 U.S.C. 3507(d). The agency solicits comments on the revision of the collection of information requirements and reduction in estimated burden hours associated with these requirements, including comments on the following items:
- Whether the collection of information are necessary for the proper performance of the agency's functions, including whether the information is useful;
- The accuracy of OSHA's estimate of the burden (time and cost) of the collection of information, including the validity of the methodology and assumptions used;
- The quality, utility, and clarity of the information collected; and
- Ways to minimize the compliance burden on employers, for example, by using automated or other technological techniques for collecting and transmitting information.
C. Proposed Information Collection Requirements
As required by 5 CFR 1320.5(a)(1)(iv) and 1320.8(d)(2), the following paragraphs provide information about the ICR.
1. Title: Hazard Communication Standard.
2. Description of the ICR: The proposal would revise the currently approved Hazard Communication ICR and change the existing collection of information requirements currently approved by OMB.
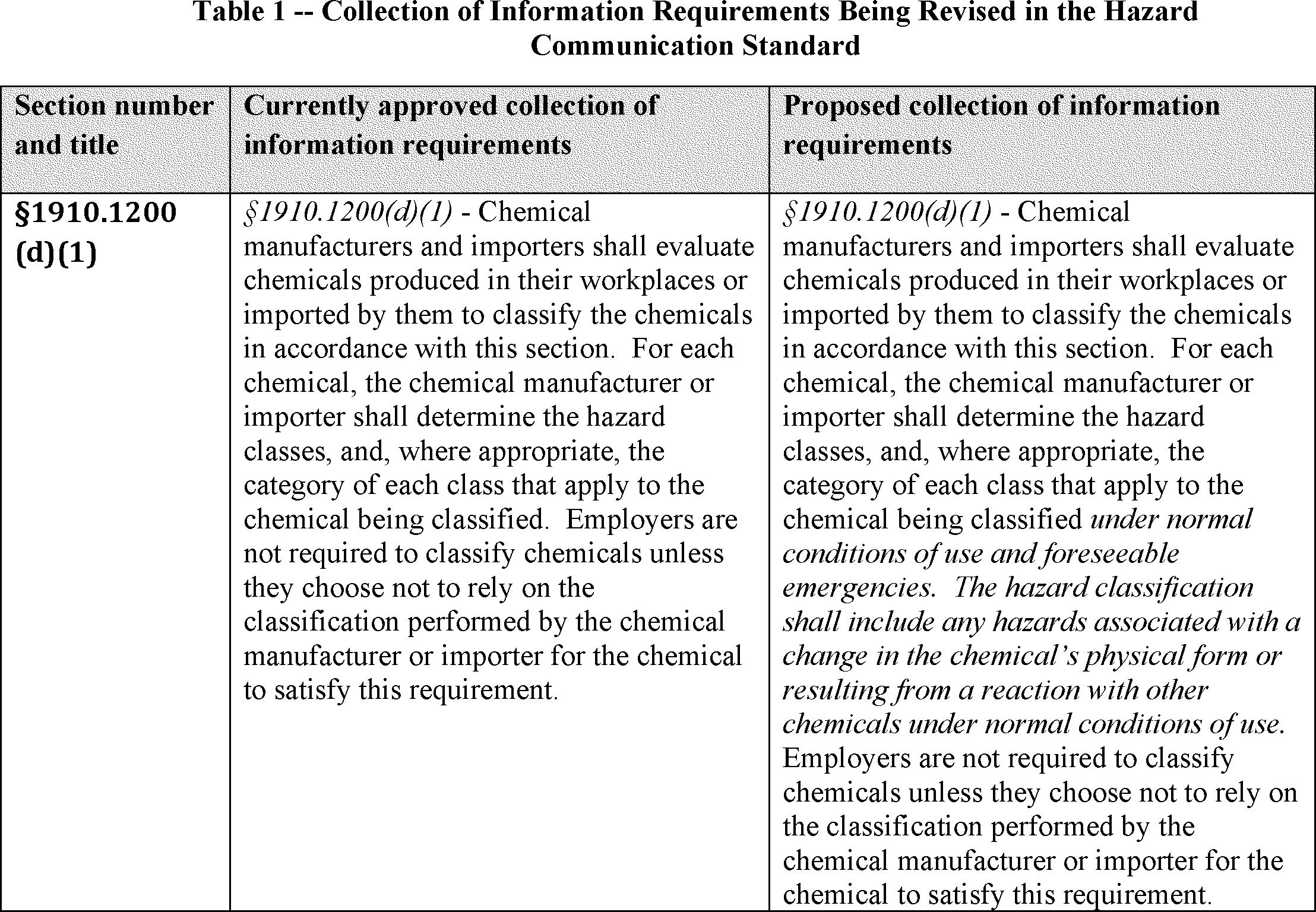
3. Brief Summary of the Information Collection Requirements: This proposal would revise and clarify the collection of information contained in the existing ICR. Specifically, OSHA is proposing to (1) add to paragraph (d)(1) that the chemical manufacturer or importer shall determine for each chemical the hazard classes, and where appropriate, the category of each class that apply to the chemical being classified under normal conditions of use and foreseeable emergencies; (2) add language to paragraph (f)(1) requiring that the chemical manufacturer, importer, or distributor ensure labels on shipped containers bear the date the chemical is released for shipment; (3) revise paragraph (f)(5) by adding two new provisions related to bulk shipments of chemicals; (4) revise paragraph (f)(11) by adding a provision related to release for shipment that requires updated labels accompany each shipment; and (5) add new labeling requirements for small containers at paragraph (f)(12). See Table 1.
Start Printed Page 9587

Start Printed Page 9588

Start Printed Page 9589

4. OMB Control Number: 1218-0072.
5. Affected Public: Business or other for-profit.
6. Number of Respondents: 2,206,700.
7. Frequency of Responses: Varies. Start Printed Page 9590
8. Number of Reponses: 74,019,955.
9. Average Time per Response: Varies.
10. Estimated Annual Total Burden Hours: 7,023,513.
11. Estimated Annual Total Cost (Operation and maintenance): $45,676,443.
D. Submitting Comments
Members of the public who wish to comment on the revisions to the paperwork requirements in this proposal must send their written comments to the Office of Information and Regulatory Affairs, Attn: OMB Desk Officer for the Department of Labor, OSHA (RIN-1218-AC93), Office of Management and Budget, Room 10235, Washington, DC 20503, email: OIRA_submission@omb.eop.gov. The agency encourages commenters also to submit their comments on the paperwork requirements to the rulemaking docket (Docket Number OSHA-2019-0001) along with comments on other parts of the proposed rule. For instructions on submitting these comments to the rulemaking docket, see the sections of this Federal Register document titled DATES and ADDRESSES. Comments submitted in response to this document are public records; therefore, OSHA cautions commenters about submitting personal information such as Social Security numbers and dates of birth.
E. Docket and Inquiries
To access the docket to read or download comments and other materials related to this paperwork determination, including the complete ICR (containing the Supporting Statement with attachments describing the paperwork determinations in detail) use the procedures described under the section of this document titled ADDRESSES.
You also may obtain an electronic copy of the complete ICR by visiting the web page at: http://www.reginfo.gov/public/do/PRAMain, scroll under "Currently Under Review" to "Department of Labor (DOL)" to view all of the DOL's ICRs, including those ICRs submitted for proposed rulemakings. To make inquiries, or to request other information, contact Ms. Seleda Perryman, Directorate of Standards and Guidance, telephone (202) 693-2222.
VII. Preliminary Economic Analysis and Initial Regulatory Flexibility Analysis
A. Introduction and Summary
Under Executive Order 12866, OMB's Office of Information and Regulatory Affairs (OIRA) determines whether a regulatory action is significant and, therefore, subject to the requirements of Executive Order 12866 and OMB review. Section 3(f) of Executive Order 12866 defines a "significant regulatory action" as an action that is likely to result in a rule that (1) has an annual effect on the economy of $100 million or more, or adversely affects in a material way a sector of the economy, productivity, competition, jobs, the environment, public health or safety, or state, local or tribal governments or communities (also referred to as economically significant); (2) creates serious inconsistency or otherwise interferes with an action taken or planned by another agency; (3) materially alters the budgetary impacts of entitlements, grants, user fees, or loan programs, or the rights and obligations of recipients thereof; or (4) raises novel legal or policy issues arising out of legal mandates, the President's priorities, or the principles set forth in Executive Order 12866. Upon review, OMB has determined that this proposed rule is a significant regulatory action ("Other Significant") under Executive Order 12866. Pursuant to the Congressional Review Act (5 U.S.C. 801 et seq.), OIRA designated that this rule is not a "major rule," as defined by 5 U.S.C. 804(2).
OSHA has made a preliminary determination that this action is not an economically significant regulatory action under section 3(f)(1) of Executive Order 12866 because it is not likely to have an annual effect on the economy of $100 million or more. This proposed rule is expected to be an Executive Order 13771 deregulatory action. Details on the estimated cost-savings of this rule can be found in the economic analysis below. Executive Order 13563 directs agencies to adopt a regulation only upon a reasoned determination that its benefits justify its costs; tailor the regulation to impose the least burden on society, consistent with obtaining the regulatory objectives; and in choosing among alternative regulatory approaches, select those approaches that maximize net benefits. Executive Order 13563 recognizes that some benefits are difficult to quantify and provides that, where appropriate and permitted by law, agencies may consider and discuss qualitatively values that are difficult or impossible to quantify, including equity, human dignity, fairness, and distributive impacts.
OSHA has prepared this Preliminary Economic Analysis (PEA), including a Preliminary Regulatory Flexibility Analysis Certification, for the proposed modifications to the HCS. Supporting materials prepared by OSHA (including spreadsheets) are available in the public docket for this rulemaking, Docket ID OSHA-2019-0001, through www.regulations.gov. OSHA invites comment on any aspects of this PEA.
In this PEA, OSHA estimates that the proposed amendments to the HCS would result in annualized net cost savings of $26.8 million at a 7 percent discount rate. Annualized at a 3 percent discount rate, OSHA estimates that the proposed amendments to the rule would lead to net cost savings of $27.5 million per year. Under a perpetual time horizon to allow for cost comparisons under Executive Order 13771, OSHA estimates that at a discount rate of 7 percent the net cost savings of the proposed amendments to the HCS would be $19.6 million per year in 2016 dollars.[8] OSHA expects that the proposed revisions to the HCS would also result in modest improvements in worker health and safety above those already being achieved under the current HCS, but the agency is unable to quantify the magnitude of these benefits.
B. Need for Regulation
Employees in work environments covered by OSHA's HCS are exposed to a variety of significant hazards associated with chemicals used in the workplace that can and do cause serious injury, illness, and death. The HCS serves to ensure that both employers and employees are provided the information they need about these chemical hazards. The current HCS contains a set of requirements for chemical products, including mandatory hazard classification, labeling requirements, provisions for providing detailed information (in SDSs), and label updating requirements. These requirements are based on Revision 3 of the GHS, which was adopted by the UN Committee and Sub-Committee of Experts on the GHS in December 2008.
OSHA has preliminarily determined that the proposed revisions to the HCS would make employers' hazard communication programs more worker-protective, efficient, and effective through standardizing practices nationally and internationally. In addition, aligning with the GHS Rev. 7 would continue to facilitate Start Printed Page 9591 international trade, as a number of U.S. trading partners are also preparing to align with the GHS Rev. 7.
The proposed revisions to the HCS include the following notable changes to improve the U.S. hazard communication system:
- Maintain alignment with the GHS
○ Adding classification categories for aerosols, desensitized explosives, and flammable gases; and
○ Updating select hazard and precautionary statements for clearer and more precise hazard information.
- Address issues identified in implementing the HCS 2012
○ Updating labeling requirements for small containers; and
○ Updating labeling requirements for packaged containers that have been released for shipment.
As discussed in Section F of this PEA, the estimated costs and cost savings resulting from the proposed revisions to the HCS consist of five main categories: (1) The cost of reclassifying affected chemicals and revising the corresponding SDSs and labels to achieve consistency with the reclassification (per proposed changes to appendix B), and the cost of revising SDSs and labels to conform with new precautionary statements and other new mandatory language in the appendices to the HCS (per proposed changes to appendices C and D); (2) the cost of management familiarization and other management-related costs (associated with all of the proposed revisions to the standard); (3) the cost of training employees as necessitated by the proposed changes to the HCS (see existing 29 CFR 1910.1200(h)(1)); (4) the cost savings resulting from the new released-for-shipment provision (proposed revisions to 29 CFR 1910.1200(f)(11)); and (5) the cost savings from limiting labeling requirements for certain very small containers (proposed 29 CFR 1910.1200(f)(12)). The first three categories are considered to be one-time costs and the last two categories are cost savings that would accrue to employers annually.
The proposed changes to the HCS would maintain the uniformity of hazard information with the GHS and would, accordingly, serve to improve the efficiency and effectiveness of the existing hazard communication system in the U.S., ensure that updated and advanced HCS methods are recognized, and reduce unnecessary barriers to trade. In short, the GHS is a "uniformity standard" for the presentation of hazard information (Hemenway, 1975, Document ID 0050). Much like other uniformity standards, such as driving on the right side of the road (in the U.S.), screw threads for fire hose connectors, "handshake" protocols for communication between computers, and, for that matter, language, the GHS provides significant efficiencies and economies.[9]
Since publication of the update to the HCS in 2012, there continues to be movement by U.S. trading partners toward maintaining standardization, consistent with the revisions in the GHS. However, OSHA does not believe that full and comprehensive standardization in accordance with the GHS, or the goal of harmonizing the U.S. system with the international one, can be achieved voluntarily in the absence of regulation.
First, the market alone will not ensure alignment with the GHS Rev. 7. In some cases (e.g., aerosols, desensitized explosives), the GHS Rev. 7 contains different hazard classes or classification criteria than the current HCS, and it would be impermissible for a manufacturer to comply with the GHS Rev. 7 rather than the criteria in the existing HCS. Moreover, making compliance with the latest revision of the GHS optional undermines the goal of harmonizing classification criteria and label elements. Second, while the costs of creating SDSs and labels are borne directly by the chemical producers, maintaining alignment with the GHS benefits the users of hazardous chemicals. These users include employers who are direct customers of chemical manufacturers, employees who use or are exposed to workplace chemicals, and emergency responders who typically have no market relationship with the chemical producers. Even if market forces could ensure the socially optimal approach to SDSs between chemical manufacturers and their customers, there are limited market forces at work between the chemical manufacturer and two key sets of users—the employees and the emergency response community. Therefore, the benefits achieved by maintaining alignment with the GHS are unlikely to be obtained in the private market without regulation.
OSHA recognizes that there will be some market pressure to align with the GHS Rev. 7 as its adoption expands internationally.[10] Some firms in the U.S. may think that they have no need to follow the GHS because they do not ship their products internationally. These firms may not realize the extent to which they are involved in international trade. There are probably few companies that have products that are never involved in international trade or that never import chemical products requiring hazard information.[11] Nonetheless, even the small percentage of U.S. companies that only conduct business domestically are required to identify and communicate hazards to workers under the HCS. Many chemical producers ship their products to distributors and are unaware of where their products are ultimately used. These distributors might well put pressure on their suppliers to maintain compliance with the GHS. Further, small companies sell chemicals to larger companies. The larger companies may use those chemicals to make other products that are exported. These larger companies might also pressure their small-firm suppliers to align with the GHS. Nevertheless, relying solely on market pressures would surely involve a long transition period, with attendant Start Printed Page 9592 losses in worker protection and production efficiencies, and it is unlikely that the market alone will ensure full alignment with the GHS for reasons described above.
The proposed changes to the HCS would involve costs and cost savings mainly for manufacturers and importers. Manufacturers and importers of chemicals would also achieve benefits—in part because they themselves benefit as both producers and users, and in part as a result of foreign trade benefits. Some manufacturers may not obtain trade benefits unless they engage in chemical export. International harmonization of hazard communication requirements may also make it easier for small companies to engage in international trade if they so desire (see additional discussion below in VII.D. Health and Safety Benefits and Unquantified Positive Economic Effects).
Of more significance to the concerns of the OSH Act, the proposed changes would also provide health benefits from improved hazard classification and communication; although unquantified in this proposal, these benefits would include reductions in worker illnesses, injuries, and fatalities (see additional discussion below in VII.D. Health and Safety Benefits and Unquantified Positive Economic Effects).
Because many of the health and safety benefits and cost savings described in this analysis require uniformity and are dispersed among a network of producers and users, only some of which have direct market relationships with each other, OSHA believes maintaining a single, uniform standard can best achieve the full benefits available from a hazard communications system.
C. Profile of Affected Industries, Establishments, and Employees
The proposed modifications to the standard include revised criteria for classification of certain health and physical hazards; revised labeling provisions for small containers and packages that have been released for shipment; revised trade secret disclosure requirements; updates to certain aspects of SDSs and precautionary statements; and related revisions to definitions of terms used in the standard.
In this section, OSHA presents a preliminary profile of industries affected by this proposal to revise the HCS. The profile data in this section are based upon the 2012 HCS final economic analysis (FEA), updated in this PEA with the most recent data available.
As a first step, OSHA identifies the North American Industry Classification System (NAICS) industries affected by the proposed changes to the HCS. Next, OSHA provides statistical information on the affected industries, including the number of affected entities and establishments; the number of workers whose exposure to the chemicals subject to the HCS could result in injury, illness, or death ("affected relevant employees"); and the average revenues and profits for affected entities and establishments by six-digit NAICS industry.[12] This information is provided for each affected industry as a whole, as well as for small entities, as defined by the Small Business Administration (SBA), and for "very small" entities, defined by OSHA as those with fewer than 20 employees, in each affected industry (U.S. Census Bureau, 2020a, Document ID 0231; U.S. Census Bureau, 2020b, Document ID 0232).
The revisions to the HCS would affect establishments in a variety of different industries in which employees are exposed to hazardous chemicals or in which hazardous chemicals are produced. The proposed changes to the HCS are not expected to change the overall list of affected industries or establishments. However, the changes are expected to affect certain establishment groupings that manufacture aerosols, desensitized explosives, and flammable gases. These proposed changes are also expected to affect certain manufacturers of hazardous chemicals that are packaged in small containers and manufacturers of chemicals that are not immediately distributed after being released for shipment.
The proposed revisions define and revise specific classifications and categories of hazards, but the scope of the requirements under which a chemical (whether a substance or mixture of substances) becomes subject to the standard is not substantially different from the current version of the HCS. Therefore, OSHA believes that the revisions would have little or no effect on whether specific establishments fall within the scope of the standard. OSHA requests comments on its preliminary determinations about the scope of the proposed revisions to the HCS and the details within the industrial profile presented in this section.
OSHA's estimates of the number of employees who will require new training under the proposed revisions to the standard are based on BLS' (2020) Occupational Employment Statistics data for May 2019, specifically the estimates of the number of employees in SOC 51-0000 Production Occupations and SOC 13-1081 Logisticians working in firms in the NAICS industries that would be affected by the proposed requirements to reclassify aerosols, desensitized explosives, and flammable gases.[13] (See the analysis and discussion of training costs below in VII.F. Compliance Costs and Cost Savings.)
Table VII-1 provides an overview of the estimated numbers of firms, establishments, and employees in each covered NAICS industry; the estimated number of employees in covered occupations (e.g., logistics personnel); and the estimated numbers of affected firms, affected establishments, and affected employees in covered occupations.[14] Tables VII-2 and VII-3, respectively, provide parallel information for all affected business entities defined as small by the SBA[15] and all affected very small business entities, defined by OSHA as those with fewer than 20 employees. The data in Start Printed Page 9593 these tables update the estimates provided in the FEA in support of the 2012 HCS final rule (Document ID 0005, Section VI) and rely on the most recent comprehensive set of data (including revenues) available from the U.S. Census Bureau (2020a; 2020b).[16]
Start Printed Page 9594

Start Printed Page 9595

Start Printed Page 9596

Start Printed Page 9597
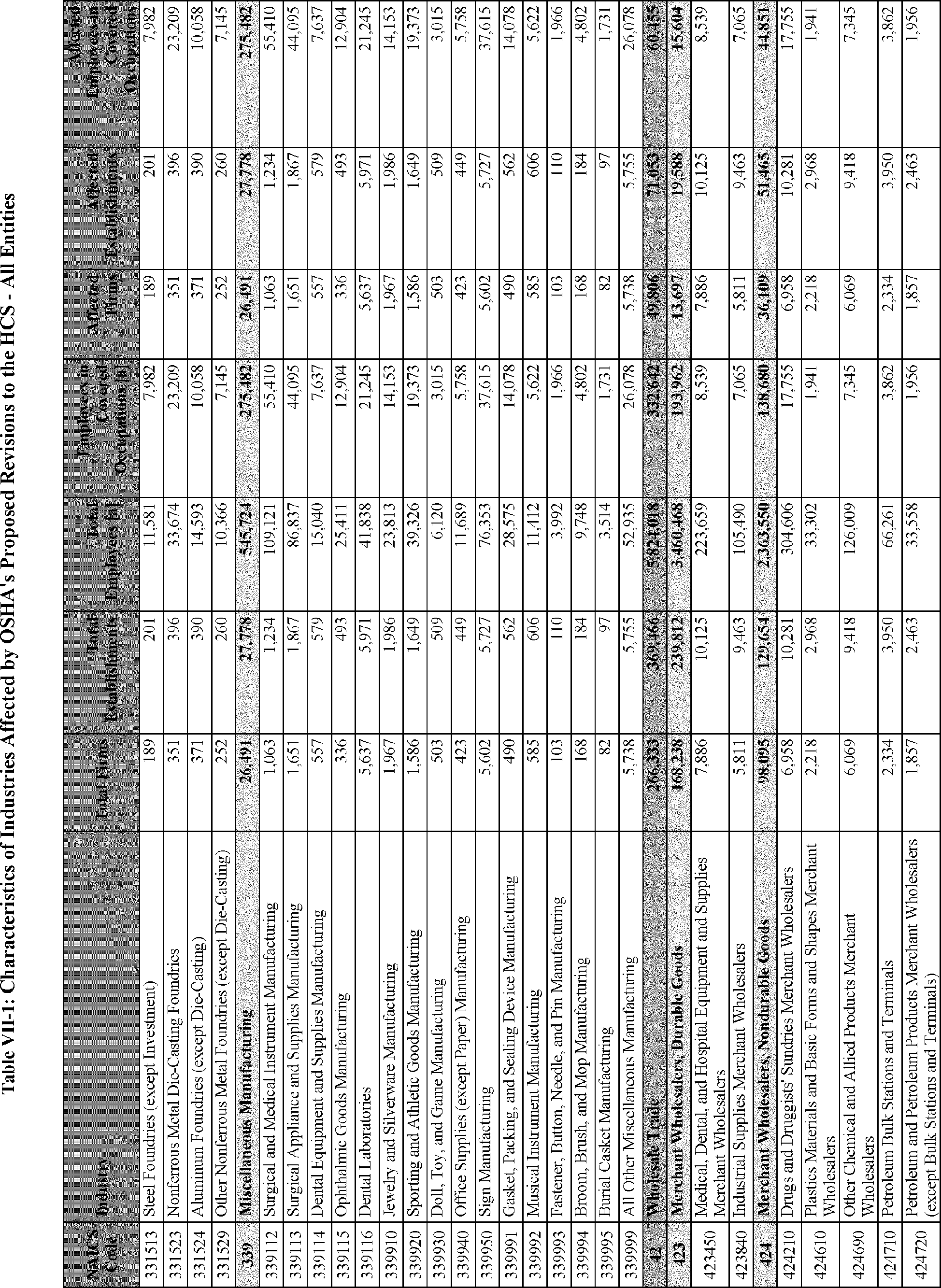
Start Printed Page 9598

Start Printed Page 9599

Start Printed Page 9600
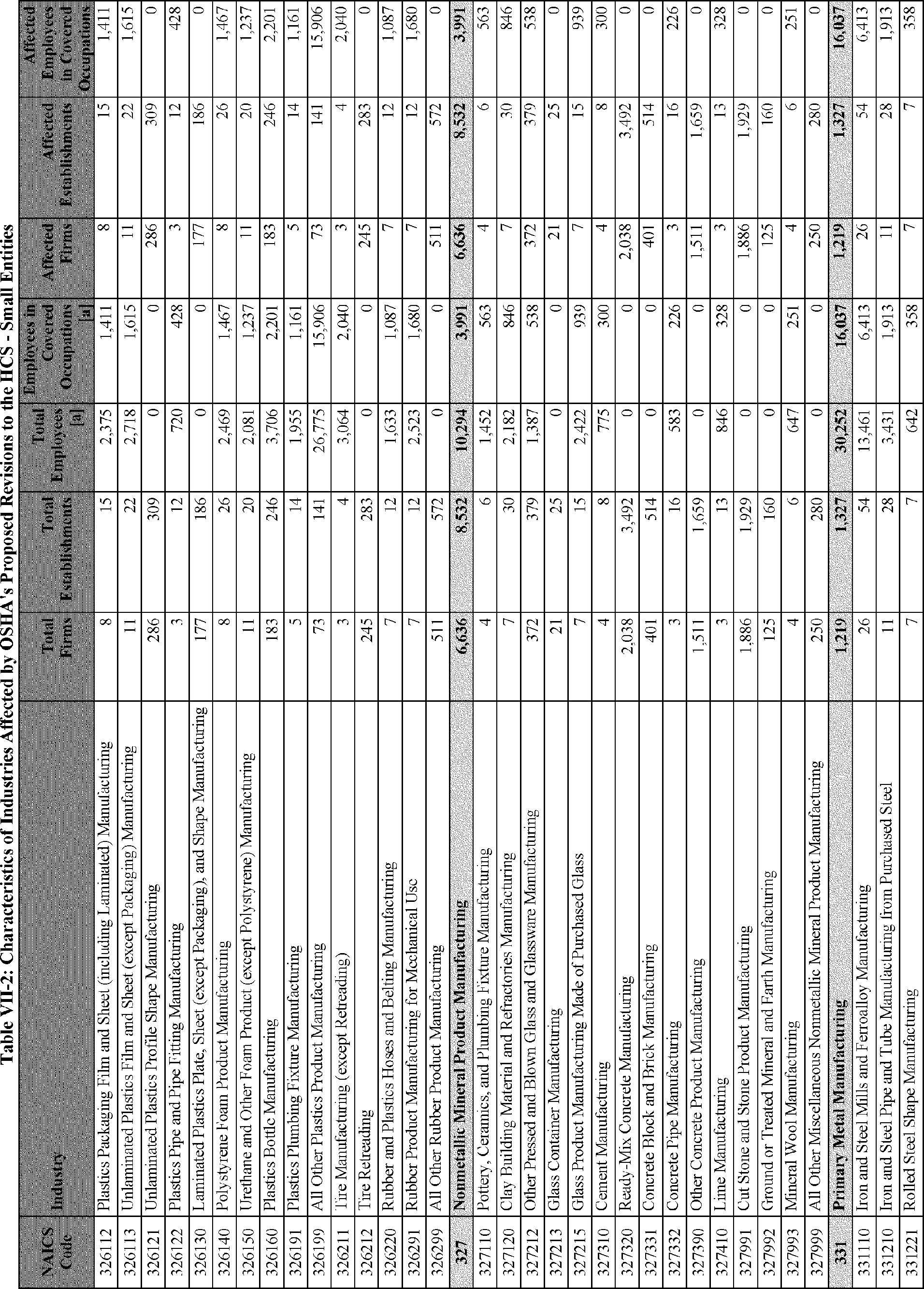
Start Printed Page 9601
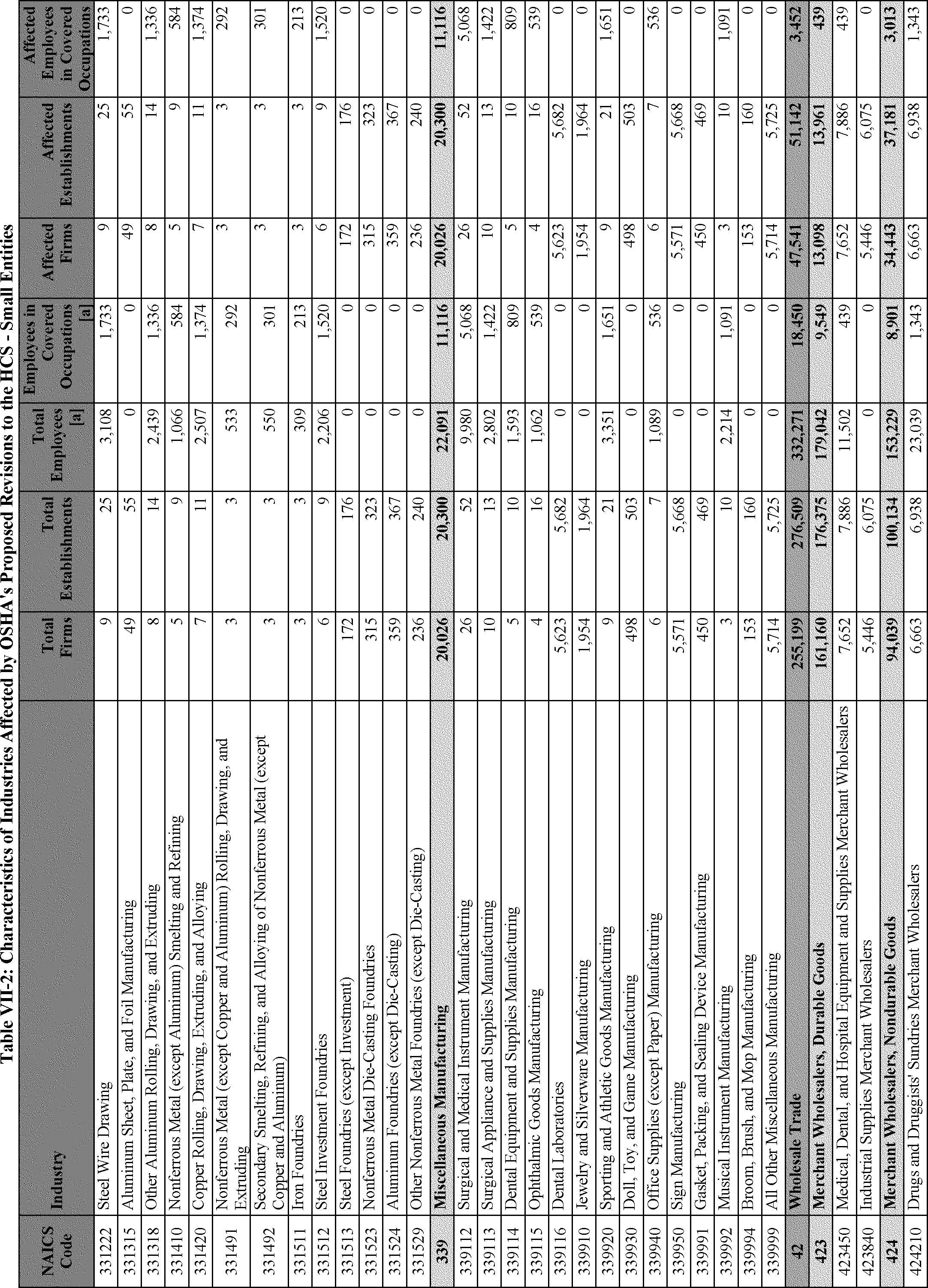
Start Printed Page 9602

Start Printed Page 9603

Start Printed Page 9604

Start Printed Page 9605

Start Printed Page 9606
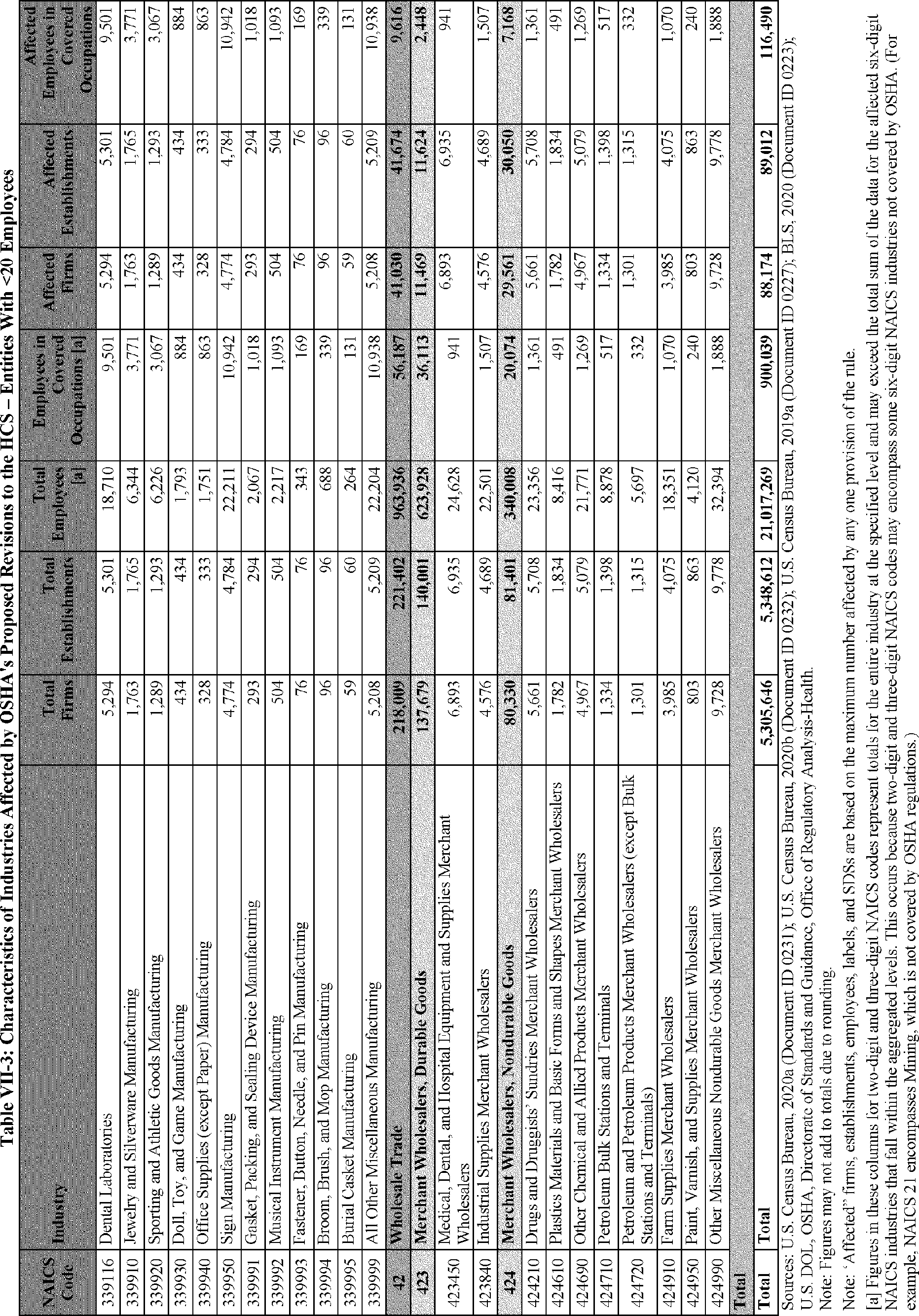
The costs and cost savings of some of the proposed provisions (new classification criteria for select hazards and labels on very small containers) are Start Printed Page 9607 driven by the number of SDSs (and labels) that manufacturers must redesign as a result of the new criteria and the number of labels on very small containers. In support of the cost analysis to follow later in this PEA, Table VII-4 presents OSHA's preliminary estimate of the number of labels per container by container size (and type).[17] Starting with the fifth row (container type: 250 ml container), Table VII-4 is drawn from data in a table (Table VI-5) presented in the FEA in support of the 2012 HCS final rule (77 FR 17640), but OSHA has updated the data to include smaller containers to permit evaluation of the impacts of the small container and very small container labeling provisions proposed in paragraph (f)(12). Also, the term "jug" has been changed to the more generic term "container." The figures in Table VII-4 are slightly different than some of the figures in Table VI-5 of the 2012 FEA due to a change in OSHA's approach to rounding and the reporting of more significant digits.
| Container type | Estimated shipment weight (lbs.) | Number of labels per containera | ||
|---|---|---|---|---|
| Minimum | Typical | Maximum | ||
| 3 ml container | 0.01 | 0.01 | 0.01 | 1.13 |
| 30 ml container | 0.06 | 0.08 | 0.13 | 1.13 |
| 60 ml container | 0.12 | 0.16 | 0.26 | 1.13 |
| 125 ml container | 0.25 | 0.33 | 0.54 | 1.13 |
| 250 ml container | 0.50 | 0.67 | 1.08 | 1.13 |
| 500 ml container | 0.92 | 1.26 | 2.08 | 1.13 |
| 1 liter container | 1.84 | 2.51 | 4.16 | 1.25 |
| 2 liter container | 3.57 | 4.92 | 8.22 | 1.25 |
| 1 gallon container | 6.83 | 9.38 | 15.63 | 1.25 |
| 2.5 gallon container | 18.00 | 24.38 | 40.00 | 1.50 |
| 5 gallon drum | 34.95 | 47.71 | 78.95 | 1.00 |
| 30 gallon drum | 202.00 | 278.56 | 466.00 | 1.00 |
| 55 gallon drum | 371.00 | 511.37 | 855.00 | 1.00 |
| 275 gallon tote | 1,830.00 | 2,531.84 | 4,250.00 | 1.00 |
| 330 gallon tote | 2,196.00 | 3,038.21 | 5,100.00 | 1.00 |
| Tank Truck—5.5k g | 34,100.00 | 48,136.79 | 82,500.00 | 0.00 |
| Tank Truck—7.0k g | 43,400.00 | 61,265.00 | 105,000.00 | 0.00 |
| Rail Car—20k g | 128,805.00 | 181,825.77 | 311,625.00 | 0.00 |
| Rail Car—30k g | 186,000.00 | 262,564.29 | 450,000.00 | 0.00 |
| Barge | 2,670,774.00 | 3,770,160.58 | 6,461,550.00 | 0.00 |
| a Assumes 8 units per package for containers smaller than 1 liter, 4 units per package for containers from 1 liter to 1 gallon in volume, and 2 units per package for 2.5-gallon containers. | ||||
| Source: U.S. DOL, OSHA, Directorate of Standards and Guidance, Office of Regulatory Analysis-Health. | ||||
As will be discussed at greater length below in Section VII.F. Compliance Costs and Cost Savings, it is OSHA's understanding that chemical manufacturers and importers periodically review, revise, and update the electronic templates they use to create SDSs and labels. Changes are made, for example, as information regarding specific hazards becomes available, new information about protective measures is ascertained, or revisions are made to product information and marketing materials. Labels and SDSs are also produced and modified when products are first introduced to the market or when products change. In this PEA, the terms "electronic templates" and "electronic files" are used interchangeably with, and as proxies for, the term "SDS." All three terms refer to the electronic files that are used to generate SDSs and labels. Table VII-5 provides, by covered NAICS industry, estimates of the total number of labels, the number of labels on very small containers (containers of 3 ml capacity or less), the total number of SDSs, and the number of labels and SDSs affected by the proposed revisions to the HCS classification criteria. The term "SDS" in the column headers and in the discussion below represents the estimated number of electronic templates (files) that are used to create SDSs and labels. The derivation of these estimates is discussed below. OSHA invites public comment on its understanding about the use of electronic template files to create SDSs and labels.
Start Printed Page 9608

Start Printed Page 9609

OSHA's estimate of the total number of SDSs per NAICS industry, as presented in Table VII-5, was developed by its contractor to support Start Printed Page 9610 the agency's FEA for the 2012 final standard.[18] The analysis started with the number of SDSs per establishment by establishment size, as originally derived in the economic analysis in support of the 2009 proposed HCS rule (Document ID 0029) using a sampling of company websites and the SDSs posted there.[19] The analysis then combined the estimated number of SDSs per establishment by establishment size with the estimated number of establishments to estimate the weighted average number of SDSs per establishment in a given NAICS industry. This estimate was then multiplied by the average number of establishments per firm to estimate the number of SDSs per firm for each NAICS industry. Multiplying by the number of firms per NAICS industry yields the total number of SDSs in each NAICS industry (as shown in Column 5 of Table VII-5). Although OSHA has preliminarily determined that this methodology remains sound, the agency invites public comment on the reasonableness of this methodology for the current analysis.
OSHA's estimate of the number of labels per NAICS industry is constructed using the same methodology developed in the 2012 HCS final rule (Document ID 0005, pp. 17634-17643), but with more recent data. The steps in the analysis, elaborated on below, can be summarized as follows:
- Begin with data on shipment weight by commodity code and shipment weight class.
- Estimate the average weight per container for containers of various sizes.
- Allocate the tons shipped in each shipment weight class for certain sizes of containers.
- Divide the tons shipped by the average container weight to estimate total containers.
- Multiply the containers by the average number of labels per container to estimate total labels.
- Allot the labels among NAICS codes using receipts data.
The label analysis begins with the U.S. Census Bureau and the U.S. Department of Transportation's jointly-produced Commodity Flow Survey (CFS) (U.S. Census Bureau, 2014a, Document ID 0024) data on shipment characteristics by commodity and shipment weight. This dataset includes the number of tons shipped for a range of shipment weight classes by Standard Classification of Transported Goods (SCTG) code. The number of tons is converted to pounds, and limited to hazardous non-consumer products (i.e., those that would have the HCS labeling).[20] This estimate is used in conjunction with another CFS dataset (U.S. Census Bureau, 2014b, Document ID 0030) that has shipment data by NAICS industry (but not by shipment weight) to divide the detailed shipment weight data into shipments coming from manufacturers and distributors.
The next step in the methodology estimated the representative weight per container for a variety of types of containers (ranging in size from a 3-milliliter vial to a rail car) and substances (such as antifreeze, diesel fuel, paint). Using representative substances, OSHA estimated the shipment weight for one container of each size as Shipment Weight = (Product Weight per gallon × Container Capacity) + Container Weight. Because of a lack of available data establishing the percentage of products shipped by container type (i.e., the breakdown of the types of products shipped by each container type), the calculation for each product and container type relied on professional judgment (by OSHA and its economic contractor, ERG) to select a "typical" product weight per gallon and container weight for each container type. Next, the analysis estimated shipment weight per container by multiplying the average product weight per gallon times the number of gallons per container, plus the container weight.
To convert the CFS data on tons (or pounds) shipped by container size into a number of containers, the analysis estimated the percentage of each shipment class likely to be shipped in certain sizes of containers. Shipments of lower weights are generally estimated to be shipped in smaller containers, and vice versa. Then the total non-consumer hazardous pounds shipped (from the CFS data) was multiplied by the estimated percentage shipped in each container type to yield the number of non-consumer hazardous pounds in each container type. Finally, the non-consumer hazardous pounds in each container type was divided by the average weight per container type to yield an estimate of the total number of containers.
To estimate the number of labels that would be used on these containers, the analysis first estimated the average number of labels on a single container for each container size (from Table VII-4 above). As previously noted, these estimates account for the fact that some containers have outer packaging that would require an additional label under this proposed rule (e.g., kits containing containers less than 100 ml where tags and fold out labels are infeasible) or are shipped with several containers grouped into a single outer container with a label. This average number of labels per container for each shipment size class was then multiplied by the number of containers to estimate the total number of labels.
The final step in the analysis was to allocate the number of labels shipped from SCTG codes to NAICS codes. The NAICS-to-SCTG mapping was adapted from the mapping used in the FEA in support of the 2012 HCS final rule analysis, but with NAICS categories updated from 2007 to 2017 categories. U.S. Census (2020a; 2020b) Statistics of U.S. Businesses data was used to estimate each NAICS industry's share of total receipts for the SCTG code with which it corresponds, and then the number of labels in each SCTG was allocated proportionally. (This calculation was performed separately for shipments from manufacturers and from distributors for purposes of estimating cost savings due to the proposed released-for-shipment provision in paragraph (f)(11)). This resulted in the estimated number of labels shown in Column 3 of Table VII-5.[21]
To estimate the number of labels on very small containers (those on containers with a volume capacity of 3 ml or less), the same analysis was performed, but it was limited to containers in that size range. The resulting estimates of the number of Start Printed Page 9611 labels on very small containers is shown in Column 4 of Table VII-5.
Not every SDS and label, and not every label on very small containers, would be affected by the proposed rule. Only SDSs and labels for certain products (aerosols, desensitized explosives, and flammable gases) would be affected by the new classification criteria. Only certain very small containers would be covered by proposed paragraph (f)(12)(iii), which would eliminate some labeling requirements in certain circumstances. In particular, under proposed paragraph (f)(12)(iii), only a product identifier would be required on the immediate outer package of very small containers (3 ml or less) where the manufacturer, importer, or distributor can demonstrate that a label would interfere with the normal use of the container and that it is not feasible to use pull-out labels, fold-back labels, or tags containing the full label information. Thus, in addition to the estimated total number of SDSs, labels, and labels on very small containers, Table VII-5 shows the number of each estimated to be affected by this proposed rule.[22]
Tables VII-6 and VII-7, respectively, provide information on total numbers of SDSs, labels, and labels on very small containers, and on the numbers of SDSs and labels (including labels on very small containers) affected by reclassification and the provisions for labels on very small containers, for all covered small entities and very small entities.
Start Printed Page 9612

Start Printed Page 9613

Start Printed Page 9614

Start Printed Page 9615
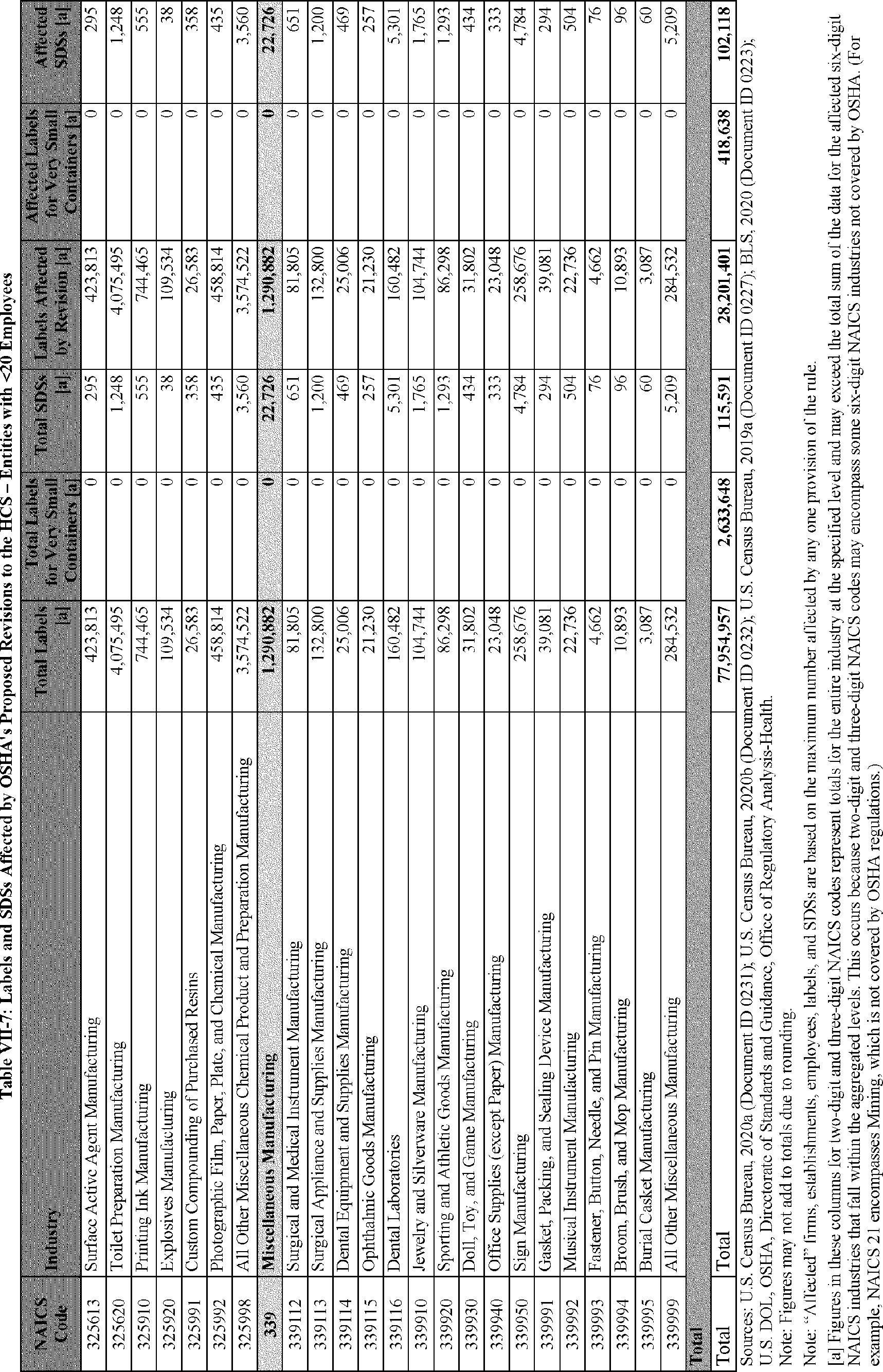
Table VI-8 shows average estimated profit rates for affected NAICS industries based on IRS (2016) SOI Tax Stats—Corporation Source Book profit data for each of the 14 years 2000- Start Printed Page 9616 2013.[23] Table VII-9 presents estimates of total revenues and total profits by NAICS industry code for all entities, small entities, and very small entities affected by this proposed rule. OSHA calculated total profits per NAICS industry by multiplying the average profit rate (NAICS industry) (IRS, 2016, Document ID 0004) by total revenues (NAICS industry) (U.S. Census Bureau, 2020a, Document ID 0231; U.S. Census Bureau, 2020b, Document ID 0232).
Table VII-10 shows, by NAICS industry code, OSHA's best estimates of the percentage of establishments or entities estimated to be affected for each element of the proposed revisions to the HCS that is projected to result in costs (see Section VII.F. Compliance Costs and Cost Savings in this PEA for an explanation of the cost categories presented in this table).[24]
Finally, Table VII-11 summarizes key estimates for the combined covered industries, labels, and SDSs affected by this proposed rule. The data in this table are drawn from profile tables presented earlier in this PEA and summarize both the magnitude of the global profile metrics (within the scope of Federal OSHA jurisdiction) and the magnitude of affected inputs critical to the agency's analysis of preliminary economic impacts.
Start Printed Page 9617

Start Printed Page 9618
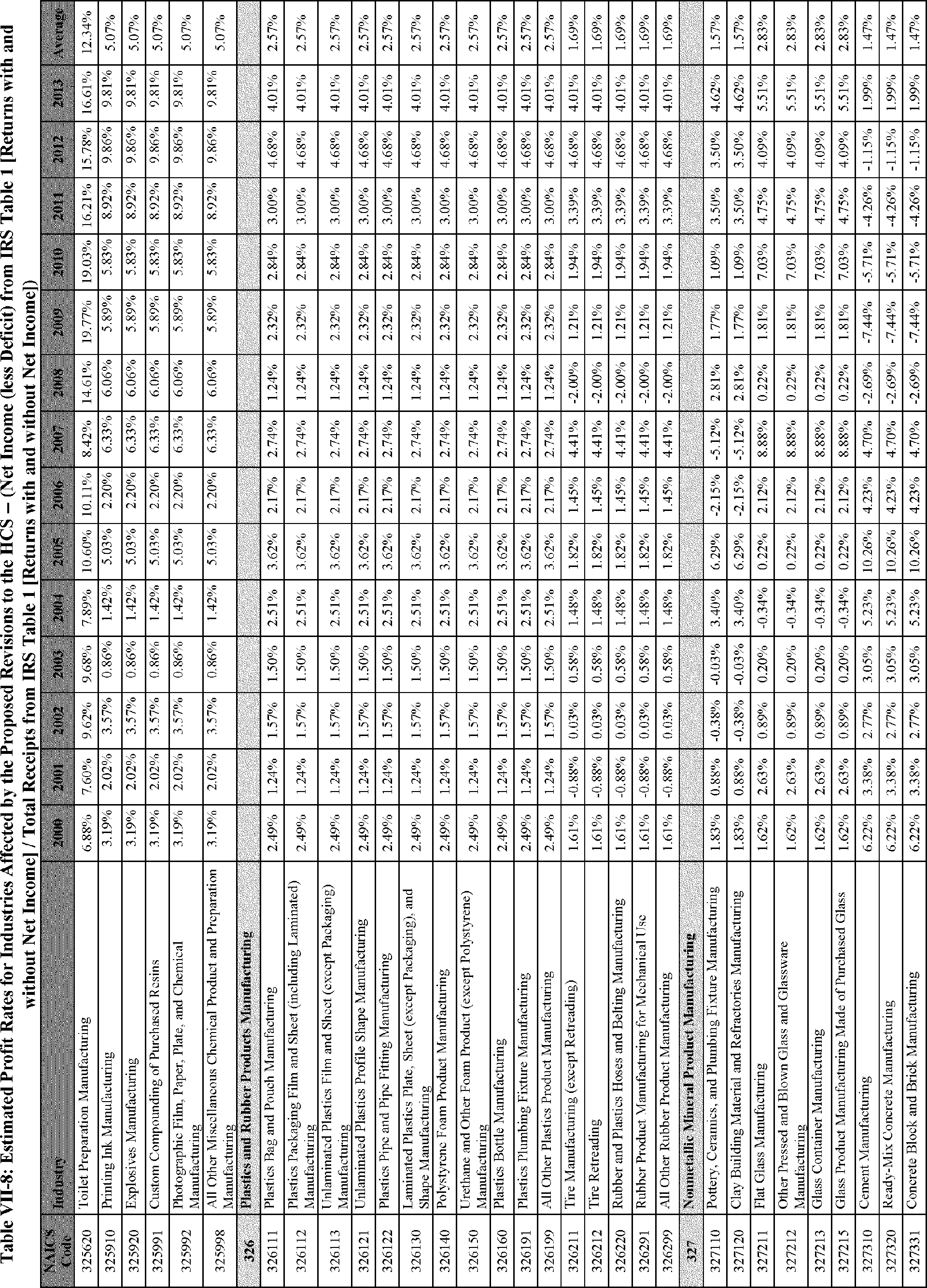
Start Printed Page 9619

Start Printed Page 9620

Start Printed Page 9621

Start Printed Page 9622
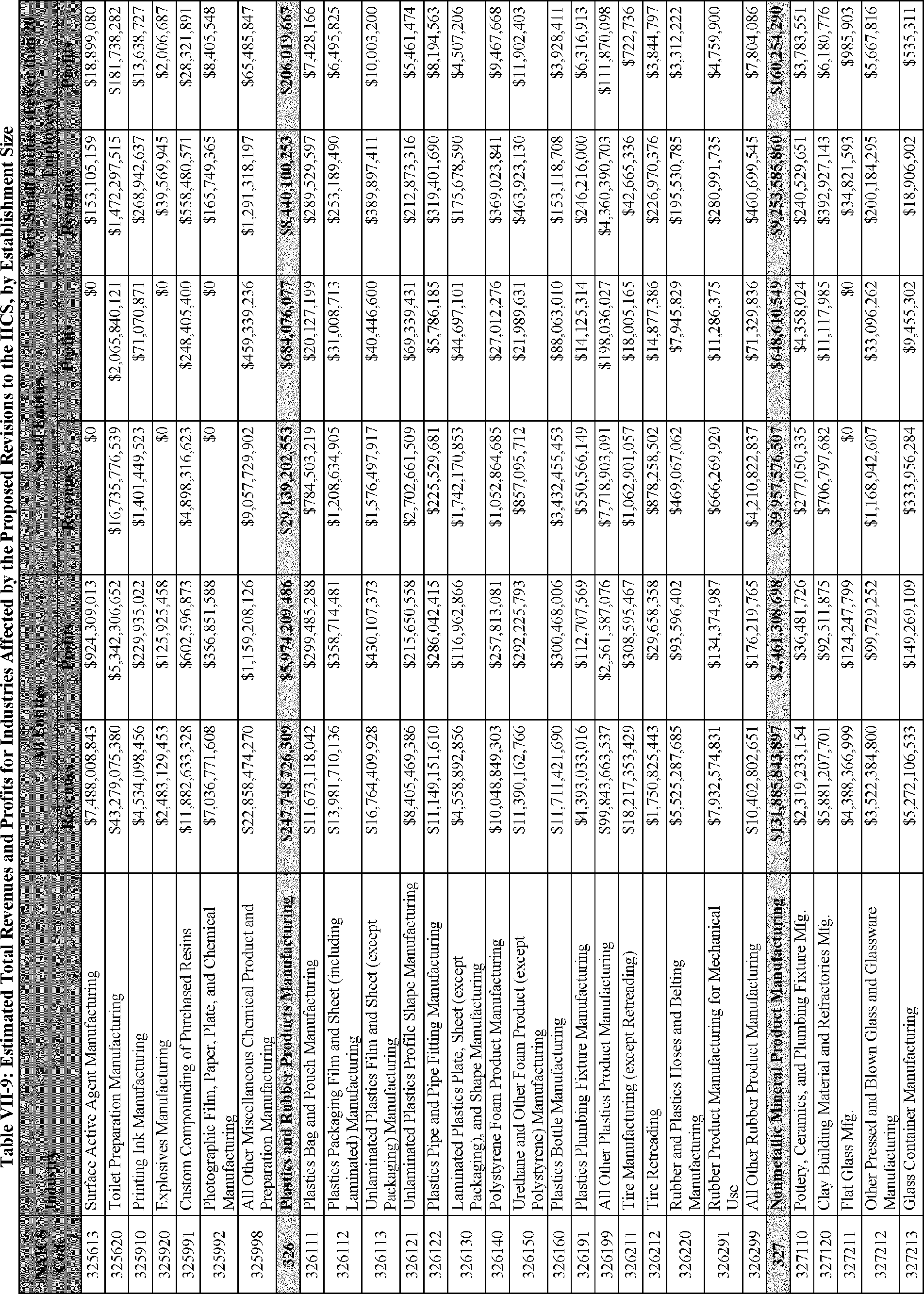
Start Printed Page 9623
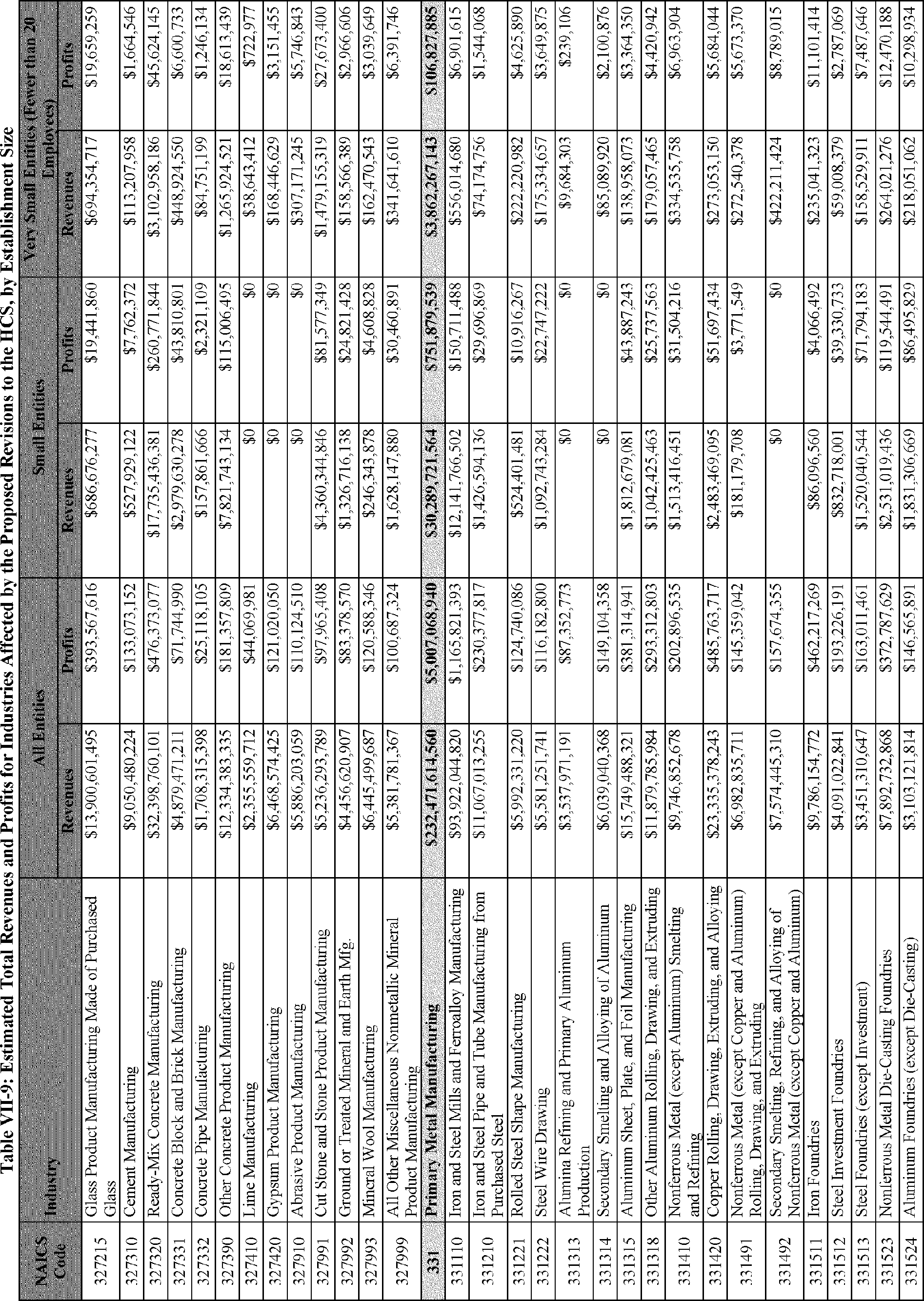
Start Printed Page 9624
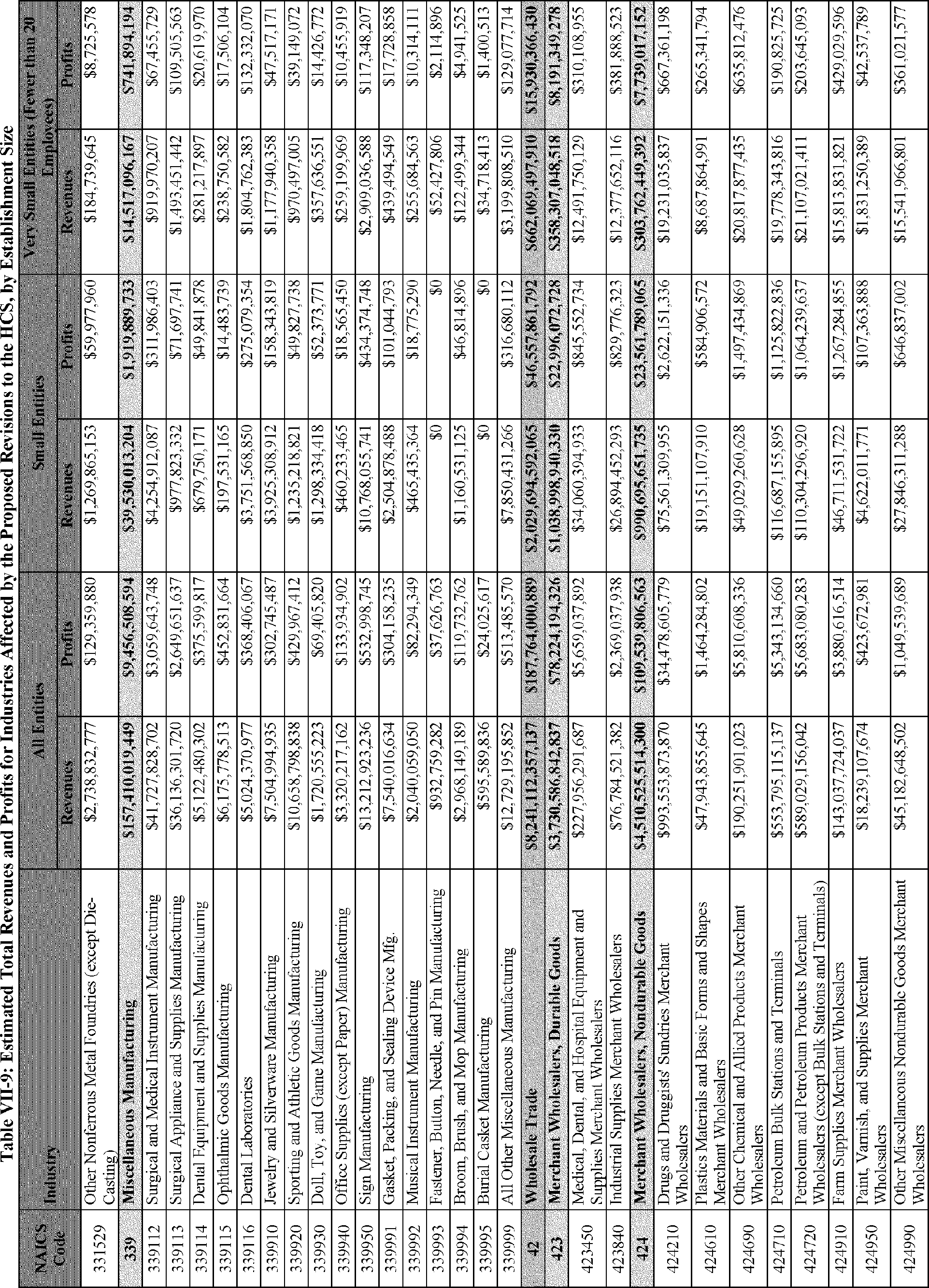
Start Printed Page 9625

Start Printed Page 9626

Start Printed Page 9627

Start Printed Page 9628

Start Printed Page 9629
| Total | Percentage affected | Affected | |
|---|---|---|---|
| Firms | 6,077,430 | 1.91 | 115,758 |
| Establishments | 7,780,863 | 1.96 | 152,427 |
| Relevant Employees | 148,004,068 | 2.82 | 4,178,738 |
| Labels Being Revised Due to Chemical Reclassification and Labels Revisions | 1,512,219,200 | 63.55 | 961,053,993 |
| Labels for Very Small Containers | 147,599,473 | 17.21 | 25,394,066 |
| Firms w/Warehoused Labels that Change | 230 | 1.00 | 2.30 |
| SDSs | 1,519,506 | 94.40 | 1,434,377 |
| Sources: U.S. Census Bureau, 2020a (Document ID 0231); U.S. Census Bureau, 2020b (Document ID 0232); U.S. Census Bureau, 2019a (Document ID 0227); BLS, 2020 (Document ID 0223); U.S. DOL, OSHA, Directorate of Standards and Guidance, Office of Regulatory Analysis-Health. | |||
| Note: Due to rounding, data derived by applying the percentages shown in the table to the figures shown in the "Total" column may not be identical to the figures shown in the "Affected" column. | |||
| a The data in this table are drawn from tables presented earlier in this PEA (for firms, establishments and employees, see Table VII-1; for labels and SDSs, see Table VII-5). | |||
D. Health and Safety Benefits and Unquantified Positive Economic Effects
As part of the rulemakings that resulted in promulgation of the original HCS in 1983, and the 1987 updates, OSHA conducted research to identify and estimate expected health and safety benefits, as described in the preambles to those final rules (48 FR 53327-53329; 52 FR 31868-31869). Combining the 1983 and 1987 rulemakings, OSHA estimated that the HCS would prevent 31,841 non-lost-workday injuries and illnesses, 20,263 lost-workday injuries and illnesses, 6,410 chronic illnesses, and 4,260 fatalities (77 FR 17621). In the 2012 final rule to modify the HCS to conform with the GHS, OSHA estimated that compliance with those revisions to the HCS would result in additional health and safety benefits equal to one percent of the previously-estimated health and safety benefits—that is, they would result in the prevention of an additional 318 non-lost-workday injuries and illnesses, 203 lost-workday injuries and illnesses, 64 chronic illnesses, and 43 fatalities annually (77 FR 17620-17624).
Relative to the HCS rulemakings that resulted in the promulgation of final rules in 1983, 1987, and 2012, these proposed revisions to the HCS are incremental and minor. Accordingly, OSHA expects that the proposed revisions to the standard will result in more modest improvements in employee health and safety than the estimated benefits OSHA attributed to the earlier rulemakings. But OSHA expects that the promulgation of the proposed revisions to the HCS will result in an increased degree of health and safety for affected employees and a corresponding reduction in the annual numbers of injuries, illnesses, and fatalities associated with workplace exposures to hazardous chemicals. Aligning with the GHS Rev. 7 will improve worker health and safety by ensuring the provision of more and better hazard information to employers and workers. For example, OSHA anticipates that the improved criteria for aerosols and flammable gases and the new hazard class for desensitized explosives, along with updated precautionary statements, will better differentiate the hazards associated with those chemicals. In addition, the proposed released-for-shipment provisions will remove the risk of injury and chemical exposures for employees who previously would have confronted the possibility of, for example, having to break down pallets of sealed, shrink-wrapped, packaged containers to replace labels when new hazards were identified.
Although OSHA expects that the proposed revisions to the HCS would reduce injuries, illnesses, and fatalities, the limited scope and nature of the changes being proposed have led OSHA to a preliminary determination that it cannot reasonably quantify an estimate of how many injuries, illnesses, and fatalities would be prevented. As the agency noted in the 2012 FEA, any assessment of benefits that are incremental to the original estimated benefits, e.g., benefits associated with minor improvements to an existing standard, broaden the range of uncertainty associated with the original estimates (77 FR 17621).[25] OSHA invites interested parties to provide comments and evidence on how the proposed revisions to the HCS are likely to affect worker safety and health.
In addition to the aforementioned health and safety benefits, OSHA expects that the proposed revisions to the HCS would result in other positive economic effects. For example, being better aligned with the GHS would help facilitate international trade, thereby enhancing competition, increasing export opportunities for U.S. businesses, reducing costs for imported products, and generally expanding the selection of chemicals and products available to U.S. businesses and consumers. As a result of the direct savings expected to result from better harmonization and the associated increase in international competition, prices for the affected chemicals and products, and the corresponding goods and services that use them, should decline, even if only to a limited extent.
Similarly, better alignment between the HCS and the GHS would have the additional benefit of meeting the international goals for adoption and implementation of the GHS that have been supported by the U.S. government.[26] Maintaining alignment with the GHS in U.S. laws and policies through appropriate legislative and Start Printed Page 9630 regulatory action was anticipated by the U.S. when it supported international mandates regarding the GHS in the Intergovernmental Forum on Chemical Safety, the World Summit on Sustainable Development, and the United Nations. It is also consistent with the established goals of the Strategic Approach to International Chemical Management that the U.S. helped to craft.[27]
E. Technological Feasibility
In accordance with the OSH Act, OSHA is required to demonstrate that occupational safety and health standards promulgated by the agency are technologically feasible. A standard is technologically feasible if the protective measures it requires already exist, can be brought into existence with available technology, or can be created with technology that can reasonably be expected to be developed. See Lead I, 647 F.2d at 1272.
OSHA has reviewed the requirements that would be imposed by the proposed rule and has assessed their technological feasibility. As a result of this review, OSHA has preliminarily determined that compliance with the requirements of the rule is technologically feasible for all affected industries.
The proposed revisions to OSHA's HCS would require manufacturers and importers to reclassify aerosols, desensitized explosives, and flammable gases in accordance with the new classification criteria and make corresponding revisions to SDSs and labels. Compliance with these requirements would mainly involve revisions to the presentation of information and is not expected to involve any technological obstacles.
The proposed changes to the requirements for the labeling of very small containers, which would eliminate full labeling requirements for some containers with a volume capacity of 3 ml or less, is expected to address current feasibility issues related to labeling these small containers. When a label would interfere with the normal use of the container, and it is not feasible to use pullout labels, fold-back labels, or tags containing full label information, the proposal would require the container to bear only the product identifier, which could be etched onto the container itself. Similarly, the proposed released-for-shipment provisions would alleviate employer concerns regarding the practicability of breaking down pallets of sealed, shrink-wrapped packaged containers to replace labels when new hazards are identified. OSHA requests public comment on any employer concerns associated with the proposed provision for labeling very small containers or with the proposed provision addressing the relabeling of containers that have been released for shipment.
OSHA has preliminarily determined that compliance with all of the requirements of the proposed revisions to the HCS can be achieved with readily and widely available technologies. No new technologies are required for compliance with the proposed modifications to the HCS. Therefore, OSHA believes that there are no technological constraints associated with compliance with any of the proposed revisions to the HCS. OSHA invites comment on these preliminary findings of technological feasibility.
F. Compliance Costs and Cost Savings
Introduction
This section presents OSHA's estimates of the costs and cost savings expected to result from the proposed revisions to the HCS. The estimated costs and cost savings are based on employers achieving full compliance with the new requirements of the proposed rule. They do not include prior costs and cost savings associated with firms whose current practices are already in compliance with the proposed requirements (where prior compliance is possible).
The estimated costs and cost savings resulting from the proposed revisions to the HCS consist of five main categories: (1) The cost of revising SDSs and labels for select hazardous chemicals to reflect chemical reclassifications (per proposed changes to appendix B) and to conform to language criteria in precautionary statements and other mandatory language (per proposed changes to appendices C and D); (2) the cost of management familiarization and other management-related costs (associated with all of the proposed revisions to the standard); (3) the cost of training employees as necessitated by the proposed changes to the HCS (see existing 29 CFR 1910.1200(h)(1)); (4) the cost savings due to the new released-for-shipment provision (proposed revisions to 29 CFR 1910.1200(f)(11)); and (5) the cost savings from limiting labeling requirements for certain very small containers (proposed 29 CFR 1910.1200(f)(12)). The first three categories are considered to be one-time costs and the last two categories are cost savings that would accrue to employers annually. Although OSHA has preliminarily determined that these are the only elements of the proposed revisions to the HCS that are expected to result in more than de minimis costs or cost savings, OSHA requests comments on whether any other proposed changes to the standard could cause employers to incur costs or obtain cost savings.
The estimated compliance costs do not include any indirect costs or impacts that may result from the reclassification or relabeling of chemicals and products already subject to the HCS, such as possible changes in production or in demand for products. Theoretically, such impacts, if any, with regard to possible changes in the uses and applications of affected chemicals, could result in costs or cost savings. OSHA expects that such effects, if any, will not be significant, but the agency would welcome input from stakeholders. This is consistent with the determination OSHA made with regard to reclassification costs for the 2012 final rule (77 FR 17625).
In order to present compliance costs and cost savings on a consistent and comparable basis across various regulatory activities, they are expressed in annualized terms. Annualized costs and cost savings represent the most appropriate measure for assessing the longer-term potential impacts of this proposed rulemaking and for purposes of comparing net costs across diverse regulations with a consistent metric. In addition, annualized net costs are often used for accounting purposes to assess the cumulative net costs of regulations on the economy or specific parts of the economy across different regulatory programs or across years.
As presented in this PEA (unless otherwise specified), a seven percent discount rate was applied to costs and cost savings arising in future years to calculate the present value of these costs and cost savings for the base year in which the standard becomes effective, and the same discount rate was then applied to the total present value costs, over a 10-year period, to calculate the annualized cost.[28] The economic effects Start Printed Page 9631 using a three percent discount rate are also provided in the Excel spreadsheets that support this PEA, which are contained in the docket (OSHA, 2020, Document ID 0049).
For the purpose of calculating loaded wage rates, OSHA did not include an overhead labor cost in the FEA in support of the 2012 HCS final standard. The Department of Labor has since determined that it is appropriate, in some circumstances, to account for overhead expenses as part of the methodology used to estimate the costs and economic impacts of OSHA regulations. For this PEA, in addition to applying fringe benefits to hourly ("base") wages, OSHA also applied an overhead rate when estimating the marginal cost of labor in its primary cost calculation.
Overhead costs are indirect expenses that cannot be tied to producing a specific product or service. Common examples include rent, utilities, and office equipment; however, there is no general consensus on the cost elements that fit the definition of overhead in the context of occupational safety and health. The lack of a common definition has led to a wide range of overhead estimates. Consequently, the treatment of overhead costs needs to be case-specific. For this PEA, OSHA has adopted an overhead rate of 17 percent of base wages, which is consistent with the overhead rate and methodology used for (1) sensitivity analyses in the FEA in support of the 2017 final rule delaying the deadline for submission of OSHA Form 300A data (82 FR 55761, 55765 (Nov. 24, 2017)); and (2) the FEA in support of OSHA's 2016 final standard on Occupational Exposure to Respirable Crystalline Silica (81 FR 16285, 16488-16492 (March 25, 2016)).[29]
To calculate the total labor cost for an occupational category, OSHA added together three components: Base wage + fringe benefits (derived as 45.8 percent of the base wage)[30] + applicable overhead costs (derived as 17 percent of the base wage). For example, the median hourly wage of an Occupational Health and Safety Specialist is $35.63. Applying a fringe markup of 45.8 percent (applied to the base wage) and an overhead rate of 17 percent (applied to the base wage) yields a fully-loaded hourly wage of $ $58.00 ($35.63 × .458 = $16.32; $35.63 × 0.17 = $6.11; $35.63 + $16.32 + $6.11 = $58.00). Note that, for this labor category, the fringe markup is equal to 28.13 percent of the fully-loaded hourly wage and that the overhead rate is equal to 10.53 percent of the fully-loaded hourly wage. Using this methodology, OSHA calculated the fully-loaded labor cost for four occupational categories: (1) Manager, Standard Occupational Classification (SOC) code 11-0000, $82.70; (2) Logistics Personnel, SOC code 13-1081, $58.51; (3) Production Worker, SOC code 51-0000, $28.18; and (4) Occupational Health and Safety Specialist, SOC code 19-5011, $58.00. (For further details, see Document ID 0049, tab "Wages".)
Table VII-12 shows the estimated annualized compliance costs and cost savings by cost category and by industry sector. All costs and cost savings are reported in 2019 dollars. As shown in Table VII-12, the total annualized net cost savings of compliance with the proposed rulemaking is estimated to be $26.8 million—consisting of about $4.4 million of annualized costs and $31.1 million of annual cost savings. Note that where tables in this PEA report estimated annualized costs, as in Table VII-12, cost savings appear as a negative number.
As shown by the three-digit NAICS Subsectors 325 (for Chemical Manufacturing) and 424 (for Merchant Wholesalers, Nondurable Goods) in Table VII-12, most of the estimated compliance costs and cost savings associated with the proposed rule would be incurred or realized by the chemical manufacturing industry and its distributors. However, the table also shows that familiarization costs would be spread across most manufacturing and wholesale industries in the U.S. economy subject to OSHA's jurisdiction, reflecting the fact that employee exposures to hazardous chemicals occur in many industry sectors.
OSHA expects that all compliance costs would be incurred in the first year, as the proposed rule would incorporate a one-year transition period into the compliance schedule for the standard. Specifically, for purposes of estimating the annualized compliance costs, OSHA assumed that the compliance costs associated with chemical reclassification, employee training, and management familiarization would be incurred in the first year following the effective date of the proposed revisions to the HCS.
Start Printed Page 9632

Start Printed Page 9633

Start Printed Page 9634

Start Printed Page 9635

Start Printed Page 9636
Estimation of Compliance Costs and Cost Savings
The remainder of his section explains how OSHA calculated the estimated compliance costs and cost savings arising from the proposed rule by describing the data and methodology used.
The major elements of the proposed revisions to the HCS that involve compliance costs or cost savings are (1) the cost of revising SDSs and labels for select hazardous chemicals to reflect chemical reclassifications (per proposed changes to appendix B) and to conform to language criteria in precautionary statements and other mandatory language (per proposed changes to appendices C and D); (2) the cost of management familiarization and other management-related costs necessary to ensure compliance with the revised standard (associated with all of the proposed revisions to the standard); (3) the cost of training employees as necessitated by the proposed changes to the HCS (see existing 29 CFR 1910.1200(h)(1)); (4) cost savings from the new released-for-shipment provision (proposed revisions to 29 CFR 1910.1200(f)(11)); and (5) cost savings from limiting labeling requirements for certain very small containers (proposed 29 CFR 1910.1200(f)(12)).
The estimated compliance costs and cost savings presented in this analysis of the proposed revisions to the HCS are based partly on analysis conducted in support of the 2012 HCS final rule (77 FR 17605-17683) and partly on new analysis prepared with the assistance of OSHA's contractor, ERG.
The estimated costs of compliance with most provisions of the proposed rule involve wages paid for the labor hours required to fulfill the requirements. In some cases, compliance could be achieved by purchasing services or products in lieu of paying employees directly. The estimated compliance costs are intended to capture the resources required for compliance regardless of how individual establishments may choose to achieve compliance.
With the exception of the proposed revision to the standard addressing precautionary statements and other mandatory language, for this cost analysis OSHA estimated a baseline compliance of zero percent. The agency's estimate of baseline compliance for the revisions in appendices C and D addressing precautionary statements and other mandatory language are discussed below in the section, Revisions to SDSs and Labels Due to Revised Precautionary Statements.
Costs Associated With Reclassifications and Revisions to Safety Data Sheets and Labels
The proposed revisions to the HCS will not change the existing requirement for firms that sell hazardous chemicals to employers to provide information about the associated hazards. Information must be presented in an SDS in the format specified in the standard, and some information must also be presented on product labels. The proposed rule would require affected chemical manufacturers to revise SDSs and labels for select hazardous chemicals to reflect chemical reclassifications (appendix B) and to conform to language criteria in precautionary statements and other mandatory language (appendices C and D). Revisions to SDSs and labels would be required under provisions in the existing HCS, which require chemical manufacturers and importers to update SDSs and labels within three months and six months, respectively, of becoming aware of significant new information regarding the hazards of the chemicals they produce or import (see 29 CFR 1910.1200(f)(11), (g)(5)).
It is OSHA's understanding that chemical manufacturers and importers periodically review, revise, and update the electronic templates they use to create SDSs and labels. Changes are made, for example, as information regarding specific hazards becomes available, new information about protective measures is ascertained, or revisions are made to product information and marketing materials. Labels and SDSs are also produced and modified when products are first introduced to the market or when products change. Therefore, there is a regular cycle of change for these documents (see 77 FR 17634-17637 in the FEA of the 2012 final rule for a discussion of factors that compel employers to update SDSs and labels voluntarily). The proposed rule would require limited changes to some SDSs and labels. Given the phase-in period for the proposed changes to the standard,[31] OSHA expects that chemical manufacturers and importers would be able to phase in revisions to their labels and SDSs in accordance with the normal cycle of change, and therefore would not need to replace existing labels or SDSs. OSHA requests comments on this preliminary assumption.
OSHA has, however, estimated costs for the time it will take to update the electronic files that will be used to generate new SDSs and labels in accordance with the proposed revisions to the HCS. OSHA developed cost estimates based on the methodology used in its FEA in support of the 2012 HCS final rule (77 FR 17634-17637). The estimated compliance costs represent the incremental costs that would be incurred to achieve compliance with the proposed rule. These estimated costs, shown below in Tables VII-13 and VII-14, would be in addition to the costs that already need to be incurred to comply with applicable requirements of the existing HCS and represent the time it would take to identify the changes that need to be made to the relevant computer files (i.e., the files that are used to generate SDSs and labels) and then to make those changes.
Producers of affected chemicals already have an obligation, under the existing HCS, to ensure that the information provided in their SDSs and labels is accurate and current (29 CFR 1910.1200(f)(2) and (g)(5)). They also are generally required to revise SDSs and labels in accordance with new information regarding hazards that may be associated with their products (29 CFR 1910.1200(f)(11) and (g)(5)). For every affected product that is newly created, reformulated, mixed with new ingredients, modified with new or different types of additives, or has any changes made in the proportions of the ingredients used, chemical manufacturers and importers are required, under the existing HCS, to review the available hazard information (29 CFR 1910.1200(d)(2)), to classify the chemical in accordance with applicable hazard criteria (29 CFR 1910.1200(d)(1)), and to develop corresponding SDSs (29 CFR 1910.1200(g)) and labels (29 CFR 1910.1200(f)). OSHA is not estimating costs for activities already required; rather, the agency is estimating costs for activities that would be newly conducted in conformance with the proposed revisions to chemical reclassifications (appendix B) and language criteria in precautionary statements and other mandatory language (appendices C and D). Start Printed Page 9637
Revisions to SDSs and Labels Due to Chemical Reclassification
The NAICS industries listed in Columns 1 and 2 of Table VII-13 are those that OSHA expects would manufacture aerosols, desensitized explosives, or flammable gases. Of course, not all chemicals covered in these NAICS industries are aerosols, desensitized explosives, or flammable gases. Column 3 of Table VII-13 reflects OSHA's judgment that approximately 50 percent of the SDSs (or more specifically, 50 percent of the electronic templates (files) that are used to produce SDSs and labels) in these NAICS industries would be affected by the proposed requirements for aerosols, desensitized explosives, and flammable gases. OSHA invites public comments on its preliminary projection that 50 percent of the electronic files for SDSs and labels would be affected in these industries.
Start Printed Page 9638

Start Printed Page 9639
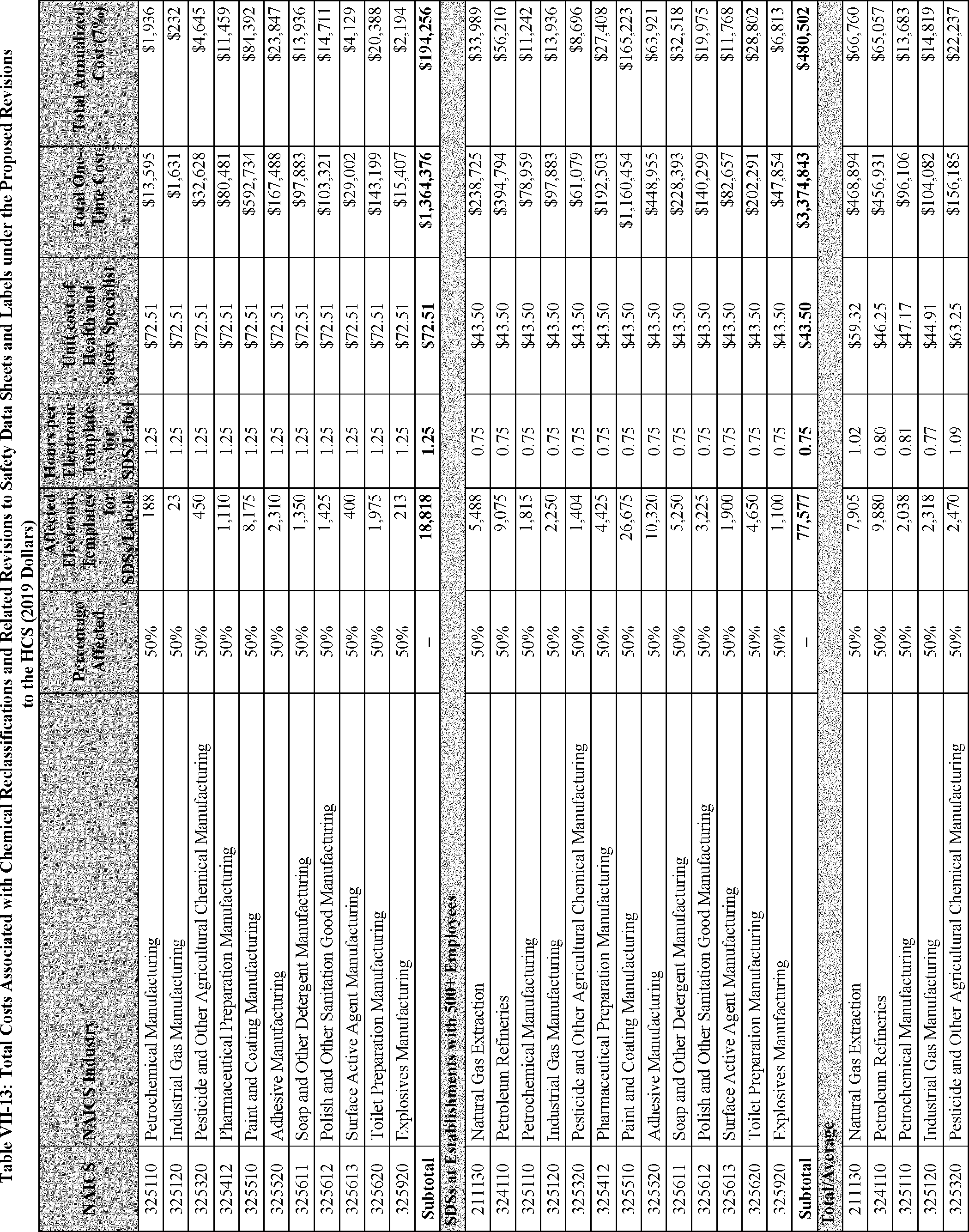
Start Printed Page 9640

OSHA derived the number of directly affected electronic files for SDSs and labels in Column 4 of Table VII-13 by applying the 50 percent factor to the overall number of affected SDSs (electronic files) from Table VII-5. For Start Printed Page 9641 example, in NAICS 211130, Table VII-5 shows the overall number of affected SDSs (technically, the number of electronic files) is 15,810. Applying a factor of 50 percent, OSHA estimated that 7,905 SDSs (electronic files) would be directly affected by the reclassification provision (see Table VII-13, NAICS 211130 within the section "Total/Average"). All of the estimates of directly affected SDSs (electronic files) presented in Table VII-13 are similarly derived from Table VII-5, but only those NAICS industries with affected SDSs (electronic files) are reported in Table VII-13.
The estimated compliance costs associated with the reclassification of hazards and related changes to SDSs and labels are directly related to the number of chemicals for which electronic files will need to be updated in order to prepare updated SDSs and labels. OSHA developed estimates of the number of potentially affected SDSs for each of the industries producing the corresponding chemicals and products (based on estimates of the total number of SDSs (and the supporting electronic files) by industry as shown in Table VII-5 of this PEA). OSHA expects downstream users, distributors, and wholesalers would continue to rely on SDSs and labels provided by manufacturers to fulfill their obligations under the OSHA standard, and would not incur costs associated with chemical reclassification under the proposed revisions to the HCS. It is OSHA's understanding that this has been the practice for decades.
Table VII-13 also contains estimates of the amount of time OSHA expects it will take to update electronic files for SDSs and labels under the proposed revisions to the standard. OSHA believes that the estimates provided in Table VII-13 are reasonable because they reflect only the incremental time needed to identify affected labels and SDSs (electronic files) and to update electronic files through modification of the templates that are used to prepare labels and SDSs, without allocating costs to any time that would be spent updating files in the absence of any revisions to the HCS.
OSHA also believes that the estimated time to update SDSs and labels (electronic files) used in this analysis represents a reasonable average for most chemicals. In the FEA in support of the 2012 HCS final rule (77 FR 17635-17637), OSHA estimated that a Health and Safety Specialist would spend between three and seven hours per SDS requiring reclassification—with smaller entities, having fewer SDSs, incurring larger costs per SDS. The revisions to the HCS currently being proposed are significantly more limited in scope than the 2012 final rule, with fewer affected hazard categories and more limited changes; however, the proposed revisions to the standard still present opportunities for scale efficiencies in reclassification. As a result, OSHA estimates that a Health and Safety Specialist would spend about 25 percent as much time to reclassify a chemical as OSHA estimated for the 2012 HCS rule—depending on establishment size, from 0.75 hours to 1.75 hours per SDS (electronic file) requiring reclassification (1.75 hours per SDS for establishments with fewer than 100 employees; 1.25 hours per SDS for establishments with 100-499 employees; and 0.75 hours per SDS for establishments with 500 or more employees).[32] At a loaded hourly wage (including overhead) of $58.00 for a Health and Safety Specialist, this would result in unit costs of $101.51, $72.51, and $43.50 per SDS for small, medium, and large establishments, respectively. Multiplying these unit costs by the estimated number of affected chemicals (i.e., electronic files) and summing the totals yields an undiscounted one-time estimated cost of $6.4 million for affected employers to comply with this provision. Annualizing this one-time cost using a 7 percent discount rate over a 10-year period results in estimated annualized costs of approximately $915,095 for reclassification in accordance with the criteria specified in the proposed revisions to the HCS. OSHA invites interested parties to comment on these cost estimates and the assumptions underlying them.
Revisions to SDSs and Labels Due to Revised Precautionary Statements, etc.
The proposed revisions to the HCS would require establishments to revise their electronic templates for SDSs and labels to conform to formatting and language criteria in precautionary statements and other mandatory language specified in appendices C and D. Under the proposed changes to the standard, affected establishments would have to update labels and SDSs for select hazardous chemicals to include updated signal word(s), hazard statement(s), pictogram(s), and precautionary statement(s) for each hazard class and associated hazard category (see existing 29 CFR 1910.1200(f) and (g)). The modification of SDSs and labels under the revisions proposed in appendices C and D would involve conforming to formatting and language standards, but would not require any additional testing, studies, or research. As previously stated, OSHA believes that chemical manufacturers and importers generally review, revise, and update their electronic templates for SDSs and labels periodically, such that there is a regular cycle of change for these documents.[33] The proposed changes to the appendices would require only limited changes to the electronic content of SDSs and labels, and, as explained previously, OSHA expects that the phase-in period for the proposed changes to the standard would allow chemical manufacturers and importers to take advantage of the normal cycle of change to phase in the revisions to their labels and SDSs, and therefore that it would not be necessary to replace existing labels or SDSs.[34] OSHA requests comments on this preliminary assumption.
The estimated compliance costs for revising electronic templates for SDSs and labels to conform to formatting and language criteria in precautionary statements and other mandatory language specified in the proposed revisions to appendices C and D represent the incremental costs that would be incurred to achieve compliance with the proposed changes to the appendices. These estimated costs, shown below in Table VII-14, would be in addition to the costs that are already incurred to comply with applicable requirements of the existing HCS.
Start Printed Page 9642

Start Printed Page 9643

Start Printed Page 9644
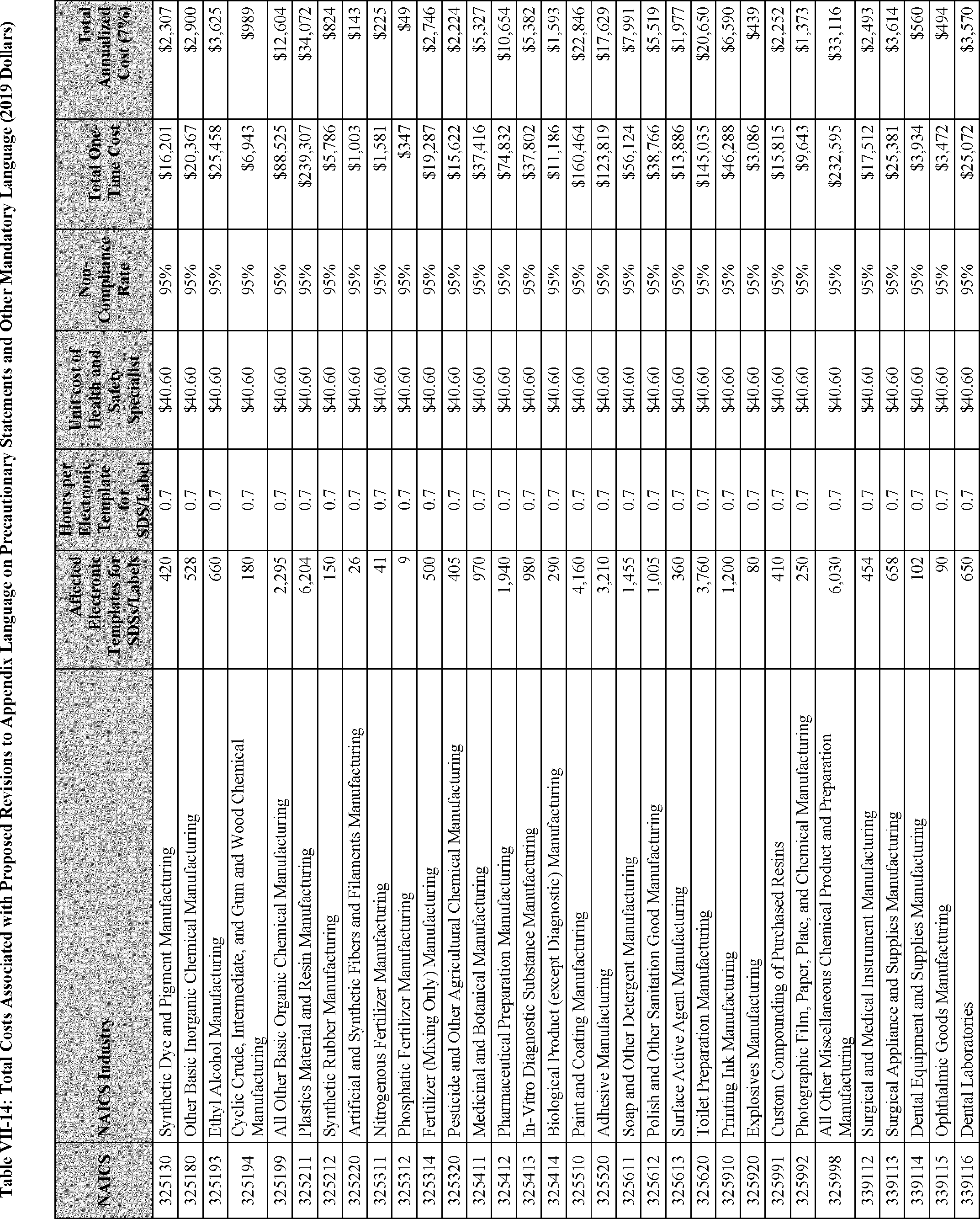
Start Printed Page 9645

Start Printed Page 9646

Start Printed Page 9647
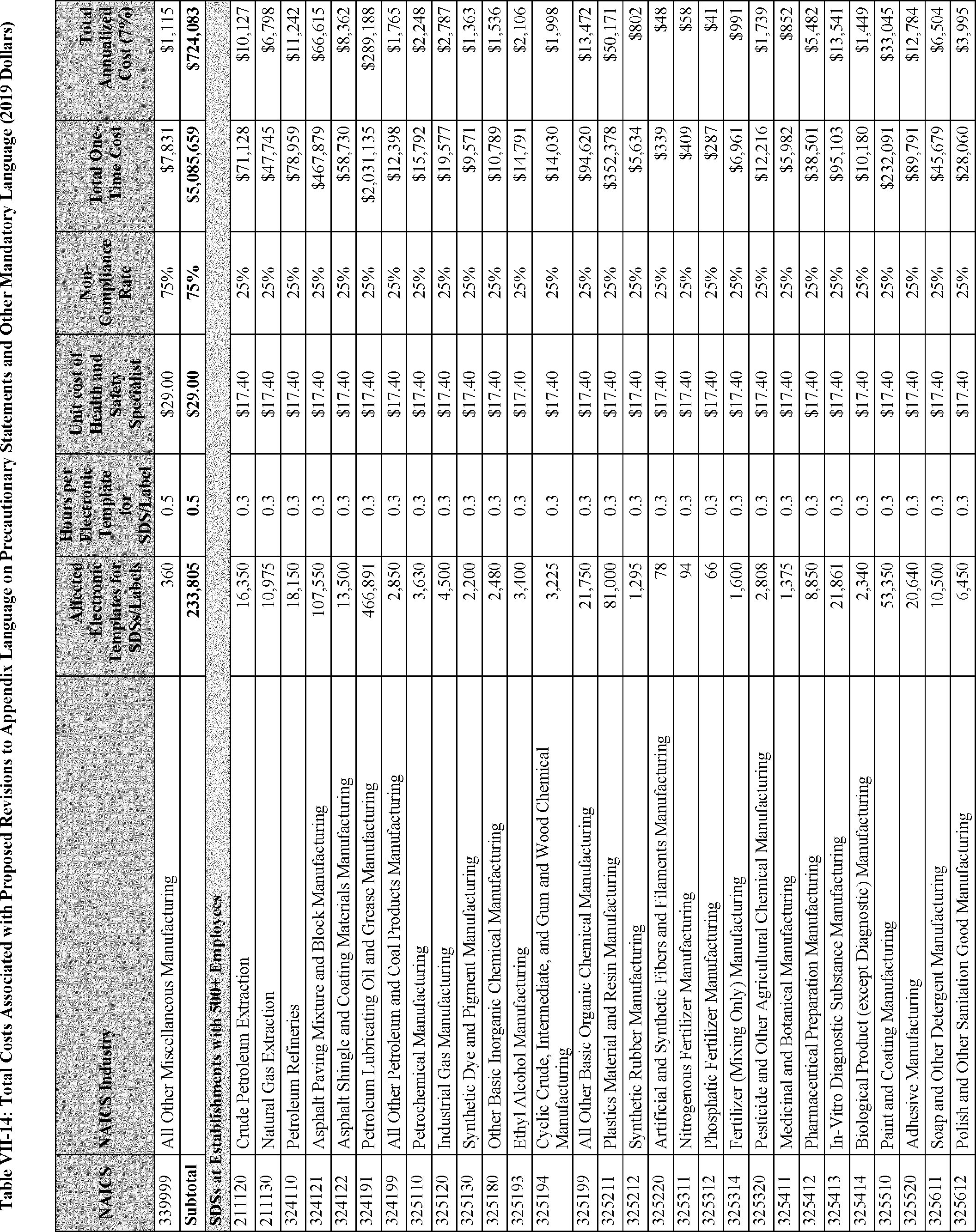
Start Printed Page 9648

Start Printed Page 9649

Start Printed Page 9650

Table VII-14 shows the estimated costs associated with modifications to electronic templates for SDSs and labels to conform to formatting and language Start Printed Page 9651 criteria in precautionary statements and other mandatory language specified in the proposed revisions to appendices C and D by NAICS industry and establishment size. The NAICS industries listed in Columns 1 and 2 of Table VII-14 are those that OSHA expects would need to update SDSs and labels under the proposed revisions to appendices C and D. The industries included are the ones OSHA identified as incurring costs for SDSs in the FEA in support of OSHA's 2012 HCS final rule (77 FR 17644-17650). The estimated costs associated with the proposed revisions to the appendices are directly related to the number of SDSs (or, in other words, the number of electronic templates) affected. These numbers were previously derived and presented in Tables VII-5, VII-6, and VII-7.[35]
OSHA estimates that the time needed to revise electronic templates for labels and SDSs to comply with the proposed revisions to appendices C and D would vary by establishment size and would be equal to 10 percent of the unit time (from 3 to 7 hours per SDS (electronic template)) estimated in the 2012 FEA (77 FR 17635-17637), as the changes the proposed revisions would require are relatively minor in comparison to the types of changes costed in 2012.[36] As shown in Column 4 of Table VII-14, OSHA estimates that Health and Safety Specialists would spend 0.7 hours per SDS (electronic template) in small establishments with fewer than 100 employees; 0.5 hours per SDS in medium establishments with 100 to 499 employees; and 0.3 hours per SDS in large establishments with 500 or more employees to comply with the proposed mandatory changes to appendices C and D. Multiplying these labor burdens by the loaded hourly wage of $58.00 results in unit costs for Health and Safety Specialists of $40.60, $29.00, and $17.40 per SDS for small, medium, and large establishments, respectively.
As in the FEA for the 2012 HCS final rule, OSHA anticipates that some manufacturers, particularly larger ones heavily involved in international trade, have already adopted the mandatory language proposed in appendices C and D. For the affected NAICS industries, OSHA estimates baseline compliance rates of 75 percent for establishments with 500 or more employees, 25 percent for establishments with 100 to 499 employees, 5 percent for establishments with 20 to 99 employees, and 1 percent for establishments with fewer than 20 employees.[37] These baseline compliance rates are the same ones OSHA used in the 2012 FEA (77 FR 17636).
Multiplying the number of affected SDSs (electronic files) by the unit cost of Health and Safety Specialists, and accounting for the relevant non-compliance rates,[38] results in an estimated total one-time cost associated with revising SDSs and labels to conform to the proposed appendix language on precautionary statements and other mandatory language. As shown in Column 7 of Table VII-14, this total one-time cost is estimated to be about $18.4 million. Annualizing this one-time cost using a 7 percent discount rate over a 10-year period results in estimated annualized costs of approximately $2.6 million for affected employers to revise SDSs and labels to comply with the proposed revisions to appendices C and D. OSHA invites interested parties to provide comments on these cost estimates and the assumptions underlying them.
The estimates of total costs in Table VII-14 are included within a broader cost category shown earlier in the aggregate costs presented in Table VII-12. Column 5 of Table VII-12 displays, by NAICS code, the combined annualized cost estimates for reclassifying chemicals (from Table VII-13) and revising SDSs and labels to be consistent with the precautionary statements and other language specified in the proposed revisions to the mandatory appendices (from Table VII-14).
Management Familiarization and Other Management-Related Costs
In order to implement the proposed new requirements in the HCS, or determine whether they need to implement any of the revisions to the standard, all employers currently covered by the standard would need to become familiar with the updates OSHA is making as part of this rulemaking. The nature and extent of the familiarization required would vary depending on the employer's business.
In the 2012 HCS final rule (77 FR 17637-17638), OSHA estimated that eight hours of time per manager, or an equivalent cost, would be associated with the necessary familiarization and implementation of revisions to hazard communication programs in affected establishments in the manufacturing sector.[39] This proposed rule would require some changes to hazard communication programs in affected establishments, but those changes would be significantly less extensive than those required by the 2012 rule. Therefore, OSHA believes that much less time would be needed for familiarization and implementation under this proposed rule than was necessary under the 2012 rule.
For the present proposed rule, OSHA estimates that management familiarization time would vary by establishment size. It would also vary depending on whether an establishment would simply be familiarizing itself with the proposed rule or would also need to take further action because it would be affected by one or more of the proposed changes to the standard. Above in Section VII.C Profile of Affected Industries, Establishments, and Employees, Table VII-10 presents, by NAICS industry, the percentage of establishments (and for training, Start Printed Page 9652 entities) expected to be affected by rule familiarization and whether those establishments or entities would incur additional costs or no additional costs—that is, whether those establishments or entities would or would not incur additional costs for revising SDSs/labels or for training employees as a result of the proposed rule.[40] In terms of manufacturing establishments that would have costs in addition to management familiarization costs resulting from the provisions of the proposed rule, OSHA estimates that there are 38,018 small establishments (those with fewer than 20 employees), 11,273 medium establishments (those with 20 to 499 employees), and 394 large establishments (those with 500 or more employees). In terms of establishments that would not have costs other than management familiarization costs resulting from the provisions of this proposed rule, OSHA estimates that there are 79,500 small establishments, 22,657 medium establishments, and 467 large establishments; their only costs associated with this proposal would be as a result of rule familiarization.[41]
To estimate unit costs, OSHA first considered establishments that would incur costs, in addition to rule familiarization costs, because of the proposed rule. As noted earlier, for the 2012 FEA OSHA applied a Manager hourly wage to estimate familiarization costs. For this PEA, because the new requirements found within this proposed standard would be significantly less extensive than those required by the 2012 rule, OSHA expects that the employer will delegate to a Health and Safety Specialist the responsibility for management familiarization of the new requirements found within this proposed standard. OSHA requests public comment on the agency's preliminary assumptions for estimating the cost of management familiarization.
For small establishments, OSHA estimated management familiarization costs of 0.5 hours of a Health and Safety Specialist's labor time. For medium establishments, OSHA estimated 2 hours of a Health and Safety Specialist's labor time. For large establishments, OSHA estimated 8 hours of a Health and Safety Specialist's labor time. Multiplying these labor burdens by the loaded hourly wage of $58.00 results in estimated management familiarization costs per establishment of $29.00, $116.01, and $464.04 for small, medium, and large establishments, respectively.
For establishments that would not incur other costs as a result of the proposed rule, OSHA estimates that rule familiarization will take half as long; in those cases, management will not need to devote as much time to considering (or making compliance decisions about) the provisions in the proposed rule that are expected to result in costs. Therefore, OSHA adopted estimates of 0.25 hours, 1 hour, and 4 hours of a Health and Safety Specialist's labor time for small, medium, and large establishments, respectively. Multiplying these labor burdens by the loaded hourly wage of $58.00 results in management familiarization costs per establishment of $14.50 for small establishments, $58.00 for medium establishments, and $232.02 for large establishments.
These management familiarization costs per establishment are multiplied by the relevant number of small, medium, and large establishments, resulting in an estimated undiscounted one-time familiarization cost of $5.2 million. Annualizing this one-time cost using a 7 percent discount rate over a 10-year period results in an estimate of annualized costs of $735,894. Table VII-15 presents the detailed unit values factoring into OSHA's estimate of management-related costs. The distribution of these management-familiarization costs by NAICS code is displayed in Column 3 of Table VII-12. OSHA invites interested parties to provide comments on these cost estimates and the assumptions underlying them.
| Small establishments (<20 employees) affected | Medium establishments (20-499 employees) affected | Large establishments (≥ 500 employees) affected | Total | |
|---|---|---|---|---|
| Directly Affected Establishments | ||||
| Total Establishments | 38,018 | 11,273 | 394 | 49,685 |
| Wage | $58.00 | $58.00 | $58.00 | |
| Hours | 0.50 | 2.00 | 8.00 | |
| Unit Cost Per Establishment | $29.00 | $116.01 | $464.04 | |
| Total One-Time Cost | $1,102,609 | $1,307,771 | $182,830 | $2,593,210 |
| Total Annualized Cost (7%) | $156,987 | $186,197 | $26,031 | $369,215 |
| Indirectly Affected Establishments | ||||
| Total Establishments | 79,500 | 22,657 | 467 | 102,624 |
| Wage | $58.00 | $58.00 | $58.00 | |
| Hours | 0.25 | 1.00 | 4.00 | |
| Unit Cost Per Establishment | $14.50 | $58.00 | $232.02 | |
| Total One-Time Cost | $1,152,841 | $1,314,209 | $108,353 | $2,575,403 |
| Total Annualized Cost (7%) | $164,139 | $187,114 | $15,427 | $366,679 |
| Total | ||||
| Total Establishments | 117,518 | 33,930 | 861 | 152,309 |
| Total One-Time Cost | $2,255,450 | $2,621,980 | $291,183 | $5,168,613 |
| Total Annualized Cost (7%) | $321,125 | $373,311 | $41,458 | $735,894 |
| Source: U.S. DOL, OSHA, Directorate of Standards and Guidance, Office of Regulatory Analysis-Health. Start Printed Page 9653 | ||||
| Note: Figures may not add to totals due to rounding. | ||||
Costs Associated With Training Employees
For this preliminary economic analysis, OSHA estimated the incremental costs to train chemical production employees who are covered by, and are already trained in accordance with, the existing standard but would need to receive additional training to become familiar with the updates to SDSs and labels for impacted aerosols, desensitized explosives, and flammable gases.[42] This analysis is described below. OSHA is not estimating any training costs for users of aerosols, desensitized explosives, or flammable gases in the workplace. OSHA does not believe that these users would need to dedicate more than a trivial amount of time to training associated with the reclassification of these chemicals. This is because the hazards associated with these chemicals have not changed. The only thing that would change under the proposed revisions to the HCS is the way the hazards are classified. For example, users of pyrophoric gases should already have received training on the fire and explosive-related hazards associated with these chemicals. At most, such users might require notification of a change in the classification of those chemicals.
Similarly, even though desensitized explosives is a new hazard classification, the explosion hazards were and are well-known and should have been included in prior hazard training. For example, should the water or other wetting solution dry out, an explosion could occur. In this case, even the hazard pictogram (flames) remains unchanged.
OSHA considered whether some increase in user training might be required for non-flammable aerosols not under pressure, since a small portion of these may not currently be classified as either flammable aerosols or gas under pressure; as noted in the Summary and Explanation section for appendix B, such aerosol containers differ from pressurized gas cylinders in terms of container characteristics and failure mechanisms. Training for non-flammable aerosols might include their revised classification and hazard avoidance measures (such as: Keep away from heat, hot surfaces, sparks, open flames and other ignition sources; no smoking; do not pierce or burn, even after use). However, based on observation of the industry over time, OSHA believes that non-flammable aerosols not under pressure are fairly uncommon and, therefore, OSHA has preliminarily concluded that the total user training time required for non-flammable aerosols not under pressure would also be negligible. The agency requests comments on its preliminary conclusions regarding training time for users of reclassified chemicals.
As discussed above, under the proposed revisions to the HCS, some chemical production employees who are covered by, and are already trained in accordance with, the existing standard would need to receive additional training to become familiar with the updates to SDSs and labels for impacted aerosols, desensitized explosives, and flammable gases. OSHA expects that the incremental training costs for these employees to become familiar with the revisions to the HCS will be small. In certain cases, affected employers will be able to integrate the necessary training into existing training programs and related methods of distributing safety and health information to employees; those employers would not incur any meaningful additional costs.
OSHA estimates that each affected chemical manufacturing firm[43] would need to devote 2.5 hours of a Health and Safety Specialist's time to preparing new training under the proposed rule, and that each affected logistics or production worker would spend 12 minutes receiving the training. Multiplying the labor burden for each labor category by the loaded hourly wages of $58.00 for a Health and Safety Specialist, $58.51 for logistics personnel, and $28.18 for production workers, results in unit costs of $145.01, $11.70, and $5.64, respectively.
Multiplying these unit costs by the 2,754 affected firms, 1,179 affected logistics managers, and 76,447 affected production workers yields an undiscounted estimated one-time cost of $843,940.[44] Annualizing this one-time cost using a 7 percent discount rate over a 10-year period results in estimated annualized costs of $120,158. The unit values that factored into OSHA's estimate of training costs are shown in Table VII-16. The distribution of these training costs by NAICS code is displayed in Column 4 of Table VII-12. OSHA invites interested parties to provide comments on these cost estimates and the assumptions underlying them.
| Health & safety specialist hours per firm to prepare training | Logistics personnel hours per emp. to receive training | Production worker hours per emp. to receive training | Total | |
|---|---|---|---|---|
| Affected Firms | 2,754 | 2,754 | ||
| Employees Needing Training | 1,179 | 76,447 | 78,489 | |
| Wage | $58.00 | $58.51 | $28.18 | |
| Hours | 2.5 | 0.2 | 0.2 | 2.9 |
| Unit Cost | $145.01 | $11.70 | $5.64 | $162.35 |
| Total One-Time Cost | $399,289 | $13,796 | $430,855 | $843,940 |
| Total Annualized Cost (7%) | $56,850 | $1,964 | $61,344 | $120,158 |
| Source: U.S. DOL, OSHA, Directorate of Standards and Guidance, Office of Regulatory Analysis-Health. | ||||
| Note: Figures may not add to totals due to rounding. | ||||
Start Printed Page 9654
Released for Shipment
In paragraph (f)(11) of the current HCS, chemical manufacturers, importers, distributors, or employers who become newly aware of any significant information regarding the hazards of a chemical must revise the labels for the chemical within six months of becoming aware of the new information and ensure that labels on containers of hazardous chemicals shipped thereafter contain the new information. OSHA proposes to modify paragraph (f)(11) such that chemicals that have been released for shipment and are awaiting future distribution need not be relabeled; however, the manufacturer or importer must provide an updated label for each individual container with each shipment.
OSHA anticipates that these proposed modifications to paragraph (f)(11) would provide cost savings to manufacturers and distributors of certain products—those with large (and typically infrequent) production runs and lengthy shelf lives (often five years or longer) that, during production, are labeled, boxed, palletized, and shipped, and then go through the distribution chain usually without the chemical contents, packaging, or label being disturbed. In Columns 1 and 2 of Table VII-17, OSHA has identified the six industries (four manufacturing and two wholesale) that it expects would be impacted by the proposed modifications to paragraph (f)(11).[45] These are primarily fertilizer manufacturers, paint manufacturers, and wholesalers of related farm and paint supplies. OSHA invites comments on whether other industries are potentially affected by this proposed modification to paragraph (f)(11) and whether there might be other health or economic effects of this proposed modification that OSHA has not considered in this proposal.
The first factor used to estimate the cost savings resulting from the proposed changes to paragraph (f)(11) is the avoided economic loss for affected manufacturers or wholesalers who would otherwise have to relabel products being held in storage. To estimate the potential economic loss avoided, OSHA relied on comments submitted to the agency by the Council of Producers & Distributors of Agrotechnology (CPDA) on April 21, 2017.[46] The CPDA comments included a summary of cost estimates associated with relabeling non-pesticide agricultural chemical products in distribution. Those estimates were obtained from an industry survey and were based on the following unit costs: Shipping costs to move product out of and back into the warehouse (for off-site package opening and replacement); relabeling space per square foot per month; safety equipment and training per employee involved in relabeling; labor and materials to break down pallets and shrink-wrap and redo product packaging in new plastic bags; and labor and materials to move liquid to new containers and dispose of old containers (CPDA, 2017, pp. 4-5, Document ID 0006).
For OSHA's purposes, the critical costing information from CPDA is the estimate of summary relabeling costs presented as a percentage of the value of the products requiring relabeling. According to the CPDA survey results, these summary costs range from 1.5 percent to 204 percent of the value of the product, depending on product type (e.g., liquid versus dry), container type (plastic bags, etc.), and the volume and value of the product (CPDA, 2017, p. 8, Document ID 0006). As a practical matter, OSHA expects that manufacturers and wholesalers would simply discard a product rather than incur relabeling costs in excess of the value of the product. Of course, there may be some disposal costs for the discarded material, but there may also be some salvage value for the improperly-labeled product. If one assumes that the disposal cost and the salvage value are relatively minor and, on net, offset each other, then the upper limit on the relabeling costs for any product would be approximately 100 percent of the value of the product. Furthermore, with an effective range of labeling costs from 1.5 percent to 100 percent of the value of the product, OSHA estimates, without further information on the distribution of the costs, that the average labeling cost would be approximately 50 percent of the value of the products requiring relabeling. While this cost estimate as a percentage of the value of the product was developed from data on relabeling non-pesticide agricultural chemical products in distribution, OSHA has assumed that this same estimate would also apply to relabeling paints and related chemical products in distribution. The agency invites comments on this assumption.
The 50 percent average cost savings estimate would apply only to those products requiring relabeling. In order to estimate the expected cost savings for all products in the NAICS codes affected by the proposed revisions to paragraph (f)(11), OSHA also needs to estimate three other factors (in addition to the average cost savings of 50 percent): (1) What percentage of the products in these NAICS industries would be warehoused for more than six months; (2) what percentage of products warehoused for more than six months would be relabeled in any particular year due to a manufacturer-initiated labeling change;[47] and (3) the percentage of all products in the NAICS industries that are covered by this proposed rule.
OSHA was unable to identify data relevant to factors (1) and (2) above and instead worked with its contractor, ERG, to develop estimates of both of these factors. For (1) above, OSHA expected that the percentage of products warehoused for more than six months would be quite low because it is expensive to hold inventory over long periods of time. Therefore, OSHA estimated that just five percent of the products in the six NAICS industries potentially impacted by the proposed modifications to paragraph (f)(11) would be warehoused for more than six months. For (2) above, OSHA anticipates that manufacturer-initiated relabeling would be rare, and estimated that only one percent of products warehoused for more than six months would be relabeled in any particular year due to a manufacturer-initiated labeling change to one or more of its chemical ingredients. See existing 29 CFR 1910.1200(f)(11). OSHA invites comments on these estimates.
For factor (3) above, OSHA assumed that 100 percent of the products in the four NAICS manufacturing industries are covered by the HCS.[48] For the two wholesale industries, however, some substantial portion of the covered products do not qualify as hazardous chemicals covered by the HCS or are not subject to the HCS labeling requirements. For NAICS 424910: Farm Supplies Merchant Wholesalers, a significant majority of the wholesale Start Printed Page 9655 supplies are non-fertilizers, such as grains (e.g., alfalfa, hay, livestock feeds) and nursery stock (e.g., plant seeds and plant bulbs). Based on data from the 2012 Economic Census,[49] ERG estimated that 41.7 percent of the wholesale supplies in NAICS 424910 would be fertilizers affected by the proposed released-for-shipment provision (Document ID 0049, tab "RF Shipment"). For NAICS 424950: Paint, Varnish, and Supplies Merchant Wholesalers, some proportion of the wholesale supply consists of non-paints and non-chemicals, such as wallpaper and painting supplies such as paintbrushes, rollers, and spray-painting equipment. Based on data from the 2012 Economic Census, ERG estimated that 77.6 percent of the wholesale supplies in NAICS 424950 would be paints and related chemicals affected by the proposed released-for-shipment provision (Document ID 0049, tab "RF Shipment"). OSHA used ERG's estimates to develop the expected cost savings attributable to the proposed revisions to paragraph (f)(11). The agency invites comments on these estimates.[50]
Column 3 of Table VII-18 shows the average product value (revenue) for each of the six NAICS industries that OSHA expects would be affected by the proposed modification to paragraph (f)(11).[51] Column 4 of Table VII-18 shows the number of affected firms (entities) for each of these six NAICS industries.[52] Column 5 of Table VII-18 shows the estimated loss avoided due to the proposed released-for-shipment provision for each of these six NAICS industries as a percentage of that industry's revenues. That percentage is the product of the four factors estimated above: (1) The costs of relabeling as a percentage of the value of the products requiring relabeling; (2) the percentage of the products in these NAICS industries that would be warehoused for more than six months; (3) the percentage of products warehoused for more than six months that would require relabeling in any particular year due to a manufacturer-initiated labeling change; and (4) the percentage of all products in the NAICS industries covered by this proposed rule.
Table VII-17 presents, by NAICS industry, these four factors and the calculated percentage loss in revenue OSHA anticipates would be avoided under the proposed released-for-shipment provision.
| NAICS | NAICS industry | Percentage cost savings | Percentage of products warehoused ≥ six months | Percentage of products warehoused ≥ six months and require relabeling | Percentage of products covered by the proposed rule | Product of percentages |
|---|---|---|---|---|---|---|
| (A) | (B) | (C) | (D) | (A), (B), (C), and (D) | ||
| 325311 | Nitrogenous Fertilizer Manufacturing | 50 | 5 | 1 | 100 | 0.03 |
| 325312 | Phosphatic Fertilizer Manufacturing | 50 | 5 | 1 | 100 | 0.03 |
| 325314 | Fertilizer (Mixing Only) Manufacturing | 50 | 5 | 1 | 100 | 0.03 |
| 325510 | Paint and Coating Manufacturing | 50 | 5 | 1 | 100 | 0.03 |
| 424910 | Farm Supplies Merchant Wholesalers | 50 | 5 | 1 | 41.70 | 0.01 |
| 424950 | Paint, Varnish, and Supplies Merchant Wholesalers | 50 | 5 | 1 | 77.60 | 0.02 |
| Source: U.S. DOL, OSHA, Directorate of Standards and Guidance, Office of Regulatory Analysis-Health. | ||||||
The estimated cost savings for each of the six affected industries arising from the proposed modifications to paragraph (f)(11) then is simply the product of Columns 3, 4, and 5 in Table VII-18. Summing the cost savings for each of the six industries yields an estimated annual cost savings of $29.8 million. OSHA requests comments on the reasonableness of this estimate and the assumptions underlying it (including the various factor percentage estimates listed in Table VII-17).
| NAICS | NAICS industry | Average product value (revenue) | Affected firms | Loss avoided as a % of revenue | Loss avoided |
|---|---|---|---|---|---|
| 325311 | Nitrogenous Fertilizer Manufacturing | $37,902,969 | 163 | 0.03 | $1,544,546 |
| 325312 | Phosphatic Fertilizer Manufacturing | 127,231,784 | 45 | 0.03 | 1,431,358 |
| 325314 | Fertilizer (Mixing Only) Manufacturing | 13,737,854 | 359 | 0.03 | 1,232,972 |
| 325510 | Paint and Coating Manufacturing | 28,813,229 | 998 | 0.03 | 7,188,901 |
| 424910 | Farm Supplies Merchant Wholesalers | 28,809,209 | 4,965 | 0.01 | 14,911,683 |
| 424950 | Paint, Varnish, and Supplies Merchant Wholesalers | 18,022,834 | 1,012 | 0.02 | 3,538,387 |
| Start Printed Page 9656 | |||||
| Total | 7,542 | 29,847,846 | |||
| Source: U.S. DOL, OSHA, Directorate of Standards and Guidance, Office of Regulatory Analysis-Health. | |||||
| Note: Figures may not add to totals due to rounding. | |||||
Labels on Very Small Containers
Proposed paragraph (f)(12), which addresses the labeling of small containers, would limit labeling requirements for chemical manufacturers, importers, or distributors where they can demonstrate that it is not feasible to use pull-out labels, fold-back labels, or tags to provide the full label information as required by paragraph (f)(1). As proposed in paragraph (f)(12)(ii), manufacturers, importers, and distributors would be able to use an abbreviated label (requiring only the product identifier, pictogram(s), signal word, chemical manufacturer's name and phone number, and a statement that the full label information is provided on the immediate outer package) on containers with a volume capacity of 100 ml or less—referred to as "small containers" in this PEA. As proposed in paragraph (f)(12)(iii), manufacturers, importers, and distributors would need to put only the product identifier on containers with a volume capacity of 3 ml or less—referred to as "very small containers" in this PEA—if they can demonstrate that any label would interfere with the normal use of the container.
Following publication of the 2012 updates to the HCS, stakeholders requested that OSHA clarify its enforcement policy on labels for small containers. In response, through letters of interpretation, OSHA adopted practical accommodations that specified: (1) The minimum information required for a label on the immediate container of the shipped chemical; and (2) the minimum information required for the outer packaging of shipped small containers (see, e.g., Collatz, 2015, Document ID 0174; Watters, 2013, Document ID 0200; Blankfield, 2017, Document ID 0170). Proposed paragraph (f)(12)(ii) would incorporate into the HCS the accommodations for small containers described in these letters of interpretation; however, the letters did not contain any guidance unique to very small containers, which would be covered by proposed paragraph (f)(12)(iii).
For costing purposes, OSHA estimates that no cost savings will arise from proposed paragraph (f)(12)(ii) (small containers); OSHA expects that employers are already benefitting from the practical accommodations on the labeling of small packages described in the aforementioned letters of interpretation. OSHA invites public comments on this preliminary determination and the magnitude of any cost savings that should be attributed to proposed paragraph (f)(12)(ii).
OSHA has estimated cost savings under proposed paragraph (f)(12)(iii) for manufacturers, importers, and distributors of very small containers (volume capacity of 3 ml or less) where the use of any label (even an abbreviated label as specified in proposed paragraph (f)(12)(ii)) would interfere with the normal use of the container and only the product identifier would be required. OSHA has preliminarily determined that affected manufacturers would fall in only a few NAICS industries: Other Basic Chemical Manufacturing, Inorganic and Organic (NAICS 325180 and 325199, respectively) and Pharmaceutical and Medical Manufacturing (NAICS 3254—encompassing 6-digit NAICS 325411, 325412, 325413, and 325414). As shown in Column 3 of Table VII-19, OSHA estimates that there are approximately 63.5 million labels on very small containers in these six 6-digit NAICS manufacturing industries that OSHA anticipates could be affected by this part of the proposed rule.[53]
Even in these six NAICS industries, however, manufacturers would not be able to take advantage of proposed paragraph (f)(12)(iii) in all cases because that provision applies only when the manufacturer, importer, or distributor can demonstrate that it is not feasible to use pull-out labels, fold-back labels, or tags containing the full label information and that even an abbreviated label would interfere with the normal use of the container. Of the 63.5 million potentially affected labels on very small containers, OSHA estimates that for only 40 percent of them, or for an estimated total of 25.4 million very small containers, would manufacturers fall under proposed paragraph (f)(12)(iii) (see Column 5 of Table VII-19 and, equivalently, Column 7 of Table VII-5).
Manufacturers with containers falling under proposed paragraph (f)(12)(iii) could expect to obtain cost savings from avoided labeling costs on very small containers (with only the product identifier required) versus the labeling costs of abbreviated labels (requiring the product identifier, pictogram(s), signal word, manufacturer's name and phone number, and a statement that the full label information is provided on the immediate outer packaging). OSHA estimates an incremental unit cost savings of $0.051 per label for very small containers.[54] That unit cost savings is expected to be net of the cost of providing a full label on the immediate outer package (containing a set of very small containers) per proposed paragraph (f)(12)(iv)(A). As shown in Table VII-19, multiplying the number of affected labels by the unit cost savings of $0.051 per label for very Start Printed Page 9657 small containers yields estimated annual cost savings of $1.3 million. OSHA invites interested parties to provide comments on these cost estimates and the assumptions underlying them.
| NAICS | NAICS industry | Labels— very small containers | Percentage of labels with cost savings | Labels w/cost savings | Annual cost savings |
|---|---|---|---|---|---|
| 325180 | Other Basic Inorganic Chemical Manufacturing | 14,768,423 | 40 | 5,907,369 | $300,518 |
| 325199 | All Other Basic Organic Chemical Manufacturing | 35,524,371 | 40 | 14,209,748 | 722,874 |
| 325411 | Medicinal and Botanical Manufacturing | 5,106,176 | 40 | 2,042,471 | 103,904 |
| 325412 | Pharmaceutical Preparation Manufacturing | 6,471,452 | 40 | 2,588,581 | 131,685 |
| 325413 | In-Vitro Diagnostic Substance Manufacturing | 501,664 | 40 | 200,665 | 10,208 |
| 325414 | Biological Product (except Diagnostic) Manufacturing | 1,113,080 | 40 | 445,232 | 22,650 |
| Total | 63,485,165 | 40 | 25,394,066 | 1,291,839 | |
| Source: U.S. DOL, OSHA, Directorate of Standards and Guidance, Office of Regulatory Analysis-Health. | |||||
| Note: Figures may not add to totals due to rounding. | |||||
Sensitivity Analysis
In this section, OSHA presents the results of a sensitivity analysis to demonstrate how robust the estimates of net cost savings are to changes in various cost parameters. In this analysis, OSHA made a series of isolated changes to individual cost input parameters in order to determine their effects on the agency's estimates of annualized net cost savings, with a seven-percent discount rate as the reference point. The agency has conducted these calculations for informational purposes only.
The methodology and calculations underlying the cost estimates associated with this rulemaking are generally linear and additive in nature. Thus, the sensitivity of the results and conclusions of the analysis will generally be proportional to isolated variations in a particular input parameter. For example, if the estimated time that employees will need to devote to attending new training doubles, the corresponding labor costs would double as well.
OSHA evaluated a series of such changes in input parameters to test whether and to what extent the general conclusions of the preliminary economic analysis held up. OSHA considered changes to input parameters that affected only costs and cost savings and determined that each of the sensitivity tests on cost parameters had only a very minor effect on total costs or net costs. On the whole, OSHA found that the conclusions of the analysis are robust, as changes in any of the cost input parameters still show significant net cost savings for the final rule. The results of the individual sensitivity tests are summarized and are described in more detail in Table VII-20.
In the first of these sensitivity tests, OSHA reduced from 1 percent to 0.5 percent its estimate of the percentage of products warehoused for more than six months that require relabeling in any particular year. The effect of this change would be to reduce by 50 percent the estimated cost savings associated with the proposed released-for-shipment provision. Table VII-20 shows that the estimated net cost savings from the proposed rule would decline by $14.9 million annually, from $26.8 million to $11.8 million annually, or by about 56 percent.
In a second sensitivity test, OSHA reversed the first sensitivity test, that is, the agency increased from 1 percent to 2 percent the percentage of products warehoused for more than six months that require relabeling in any particular year. The effect of this change would be to increase by 100 percent the estimated cost savings associated with the proposed released-for-shipment provision. Table VII-20 shows that the estimated net cost savings from the proposed rule would increase by $29.8 million annually, from $26.8 million to $56.6 million annually, or by about 112 percent.
In a third sensitivity test, OSHA reduced from 40 percent to 20 percent the percentage of very small containers that would be affected by proposed paragraph (f)(12). As shown in Table VII-20, if OSHA's estimates of other input parameters remained unchanged, the estimated net cost savings from the proposed rule would decline by $0.6 million annually, from $26.8 million to $26.1 million annually, or by about 2 percent.
In a fourth sensitivity test, OSHA applied the same rule familiarization costs to all firms regardless of whether they are affected by other provisions of this proposal, i.e., OSHA did not reduce estimated familiarization time for firms that are not affected by other parts of the proposal. The effect of this change would be to raise compliance costs for 102,624 establishments in manufacturing and wholesale trade; the estimated net cost savings from the proposed rule would be reduced by $0.4 million annually, from $26.8 million to $26.4 million annually, or by about 1 percent.
In a fifth sensitivity test, OSHA doubled the estimated labor hours assigned to revising SDSs and labels due to the reclassification of chemicals and revised mandatory language in the appendices of the HCS (from Tables VII-13 and VII-14). The effect of this change would be to double labor costs for the affected six-digit NAICS industries; estimated net cost savings would be reduced by $3.5 million annually, from $26.8 million to $23.2 million, or by 13.2 percent.
In a sixth sensitivity test, OSHA excluded overhead costs from the fully loaded hourly wage rates used throughout the PEA. Overhead costs were not applied in the 2012 FEA and this sensitivity test provides consistency with the treatment of overhead in the 2012 analysis. The effect of this change would be to remove the factor of 17 percent of base wages from the hourly costs for the four job categories used in the cost analysis. Applying this change, the estimated net cost savings from the proposed rule would increase by $0.5 million annually, or by 1.7 percent, resulting in a total estimate of annualized net cost savings of $27.2 million. Start Printed Page 9658
| Uncertainty (cost) scenarios | Change from OSHA's best estimate | Difference from proposed rule | Percentage impact on net cost savings | Net cost savings |
|---|---|---|---|---|
| Proposed Rule—OSHA's midpoint estimate | N/A | $0 | 0.0 | $26,753,711 |
| Reduce from 1 percent to 0.5 percent the percentage of products warehoused for more than six months that would require relabeling in any particular year | Halves cost savings associated with proposed released-for-shipment provision | −$14,923,923 | −55.8 | 11,829,788 |
| Increase from 1 percent to 2 percent the percentage of products warehoused for more than six months that would require relabeling in any particular year | Doubles cost savings associated with proposed released-for-shipment provision | 29,847,846 | 111.6 | 56,601,557 |
| Reduce from 40% to 20% the percentage of very small containers that would be affected by proposed paragraph (f)(12) | Halves cost savings for affected firms | −645,919 | −2.4 | 26,107,792 |
| Rule familiarization time would not be reduced for firms that are not affected by any other cost provisions; it would be identical to rule familiarization time for those that are affected by other provisions | Raises costs for the 31,577 establishments in NAICS 31-33—Manufacturing, and the 71,047 establishments in NAICS 42—Wholesale Trade not affected by other provisions | −366,679 | −1.4 | 26,387,032 |
| Doubles labor hours for the reclassification of chemicals and compliance with the new mandatory language in the appendices to the proposed standard | Doubles labor costs for the approximately 13 six-digit NAICS industries affected by proposed changes to paragraph (d) and appendices B, C, and D | −3,529,921 | −13.2 | 23,223,790 |
| Excludes overhead costs from fully loaded hourly wage rates | For the four job categories in the cost model, overhead costs (17 percent of base wages) are not applied and estimated wage rates are correspondingly lower | 458,003 | 1.7 | 27,211,714 |
| Remove the proposed provisions that result in cost savings for very small labels | Eliminates cost savings for affected employers | −1,291,839 | −4.8 | 25,461,873 |
| Eliminate the proposed released-for-shipment provisions and associated cost savings | Eliminates cost savings for affected employers | −29,847,846 | −111.6 | −3,094,135 |
| Source: U.S. DOL, OSHA, Directorate of Standards and Guidance, Office of Regulatory Analysis-Health (Document ID 0049, tab "Tables"). | ||||
Not part of this table, but discussed in A. Introduction and Summary, the agency examined the effect of lowering the discount rate for annualizing costs from 7 percent to 3 percent. Lowering the discount rate to 3 percent would yield annualized net cost savings of $27.5 million, approximately $700,000 more in annual cost savings than the net cost savings at a 7 percent discount rate.
Regulatory Alternatives
This section discusses two regulatory alternatives to the changes OSHA is proposing in this NPRM: (1) Removing the proposed changes to paragraph (f)(12) regarding labeling of very small containers, which would eliminate cost savings for manufacturers, importers, and distributors that label such containers; and (2) removing the proposed changes to paragraph (f)(11) regarding labeling of containers that have been released-for-shipment, which would eliminate cost savings for manufacturers, importers, and distributors that have such containers. In Table VII-20, each regulatory alternative is described and analyzed relative to the proposed revisions to the HCS. Midpoint estimates are presented in all cases. Under Regulatory Alternative (1) (elimination of changes related to labeling of very small containers), cost impacts total $1.3 million (4.8 percent of baseline cost savings), resulting in a reduction of estimated annualized net cost savings to a total of $25.5 million. Under Regulatory Alternative (2) (elimination of changes related to labels on packages that have been released for shipment), cost impacts on employers total $29.8 million (112 percent of baseline cost savings), resulting in an overall estimate of annualized net costs of $3.1 million.
In summary, these regulatory alternatives would result in a reduction of cost savings—a significant reduction in the case of the second alternative (resulting in positive, but modest, overall net costs). The elimination of neither alternative, however, would alter the agency's determination of economic feasibility for the proposed revisions to the HCS as a whole. Nor would the elimination of these alternatives result in a significant impact on a substantial number of small entities (see Section VII. G. Economic Feasibility and Impacts).
G. Economic Feasibility and Impacts
This section presents OSHA's analysis of the potential economic impacts of the proposed rule and an assessment of economic feasibility. A separate analysis of the potential economic impacts on small entities (as defined in accordance with SBA criteria) and on very small entities (those with fewer than 20 employees) is presented in the following section as part of the Initial Regulatory Flexibility Screening Analysis, conducted in accordance with the criteria laid out in the Regulatory Flexibility Act.
A standard is economically feasible "if it does not threaten massive dislocation to, or imperil the existence of, [an] industry." Lead I, 647 F.2d at 1265 (internal citations and quotation marks omitted). To determine whether a rule is economically feasible, OSHA begins with two screening tests to consider minimum threshold effects of the rule under two extreme cases: (1) A scenario in which all costs are passed through to customers in the form of higher prices (consistent with a price elasticity of demand of zero); and (2) a scenario in which all costs are absorbed by the firm in the form of reduced profits (consistent with an infinite price elasticity of demand).
In profit-earning entities, compliance costs can generally be expected to be absorbed through a combination of increases in prices and reductions in profits. The extent to which the impacts of cost increases affect prices or profits depends on the price elasticity of demand for the products or services produced and sold by the entity.
The price elasticity of demand refers to the relationship between changes in the price charged for a product and the resulting changes in the demand for that product. A larger price elasticity of demand implies that an entity or industry is less able to pass increases in costs through to its customers in the form of a price increase and must absorb more of the cost increase through a reduction in profits. Start Printed Page 9659
If the price elasticity of demand is zero, and all costs can be passed to customers in the form of higher prices, the immediate impact of the rule would be observed in the form of increased industry revenues. In the absence of evidence to the contrary, OSHA generally considers a standard to be economically feasible for an industry when the annualized costs of compliance are less than a threshold level of one percent of annual revenues. Common-sense considerations indicate that potential impacts of such a small magnitude are unlikely to eliminate an industry or significantly alter its competitive structure, particularly since most industries have at least some ability to raise prices to reflect increased costs and normal price variations for products typically exceed three percent a year (OSHA, 2016, Chapter VI, pp. VI-20/VI-23 and Table VI-3).[55] Of course, OSHA recognizes that even when costs are within this range, there could be unusual circumstances requiring further analysis.
If, however, there is infinite price elasticity of demand, and all costs are absorbed by affected firms, the immediate impact of the rule would be observed in reduced industry profits. OSHA uses the ratio of annualized costs to annual profits as a second check on economic feasibility. In the absence of evidence to the contrary, OSHA generally considers a standard to be economically feasible for an industry when the annualized costs of compliance are less than a threshold level of ten percent of annual profits. This is a fairly modest threshold level, given that normal year-to-year variations in profit rates in an industry can exceed 40 percent or more (OSHA, 2016, Chapter VI, pp. VI-20/VI-23 and Table VI-5).[56]
In order to assess the nature and magnitude of the economic impacts associated with compliance with the proposed rule, OSHA developed quantitative estimates of the potential economic impact of the requirements on each of the affected industry sectors. The estimated costs of compliance presented in Section VII.F of this preamble were compared with industry revenues and profits to provide a measure of potential economic impacts. Table VII-21 presents data on revenues and profits for each affected industry sector at the six-digit NAICS industry level, along with the corresponding estimated annualized costs of compliance in each sector. Potential impacts in the table are represented by the ratios of compliance costs to revenues and compliance costs to profits.
The nature of the proposed revisions to the HCS is such that all affected firms would incur some costs, but only a small subset would derive the cost savings that are monetized in this PEA (although most or all would enjoy non-monetized benefits, e.g., in foreign trade). To examine the economic impacts of the proposed revisions to the standard for those affected establishments that obtain no monetized cost savings from any of the proposed revisions to the HCS, OSHA estimated the ratio of compliance costs to revenues and the ratio of compliance costs to profits using only gross positive costs (i.e., costs exclusive of cost savings) as the numerator in the ratio. Table VII-22 presents this part of the agency's screening analysis.
Start Printed Page 9660
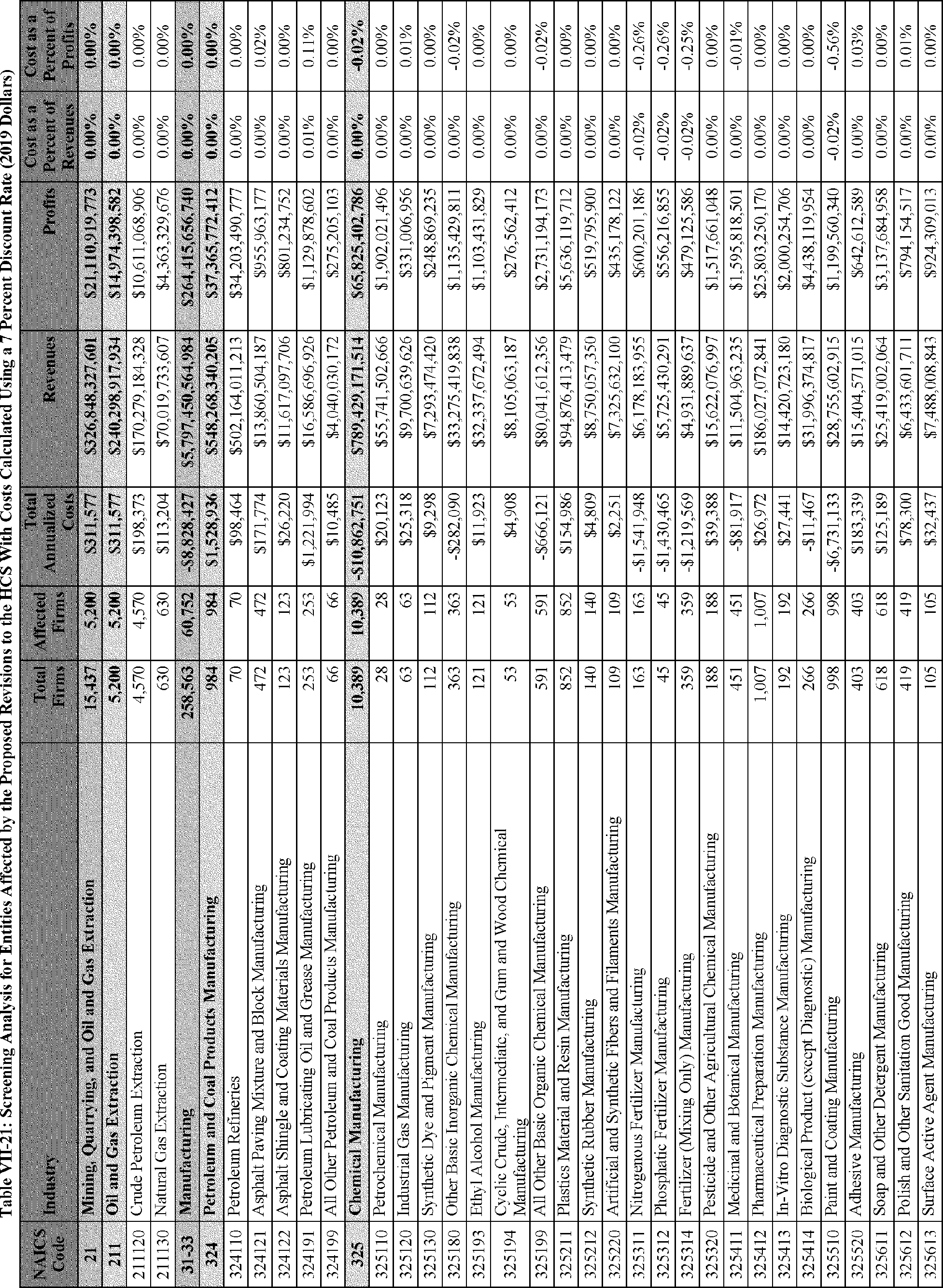
Start Printed Page 9661

Start Printed Page 9662

Start Printed Page 9663

Start Printed Page 9664

Start Printed Page 9665
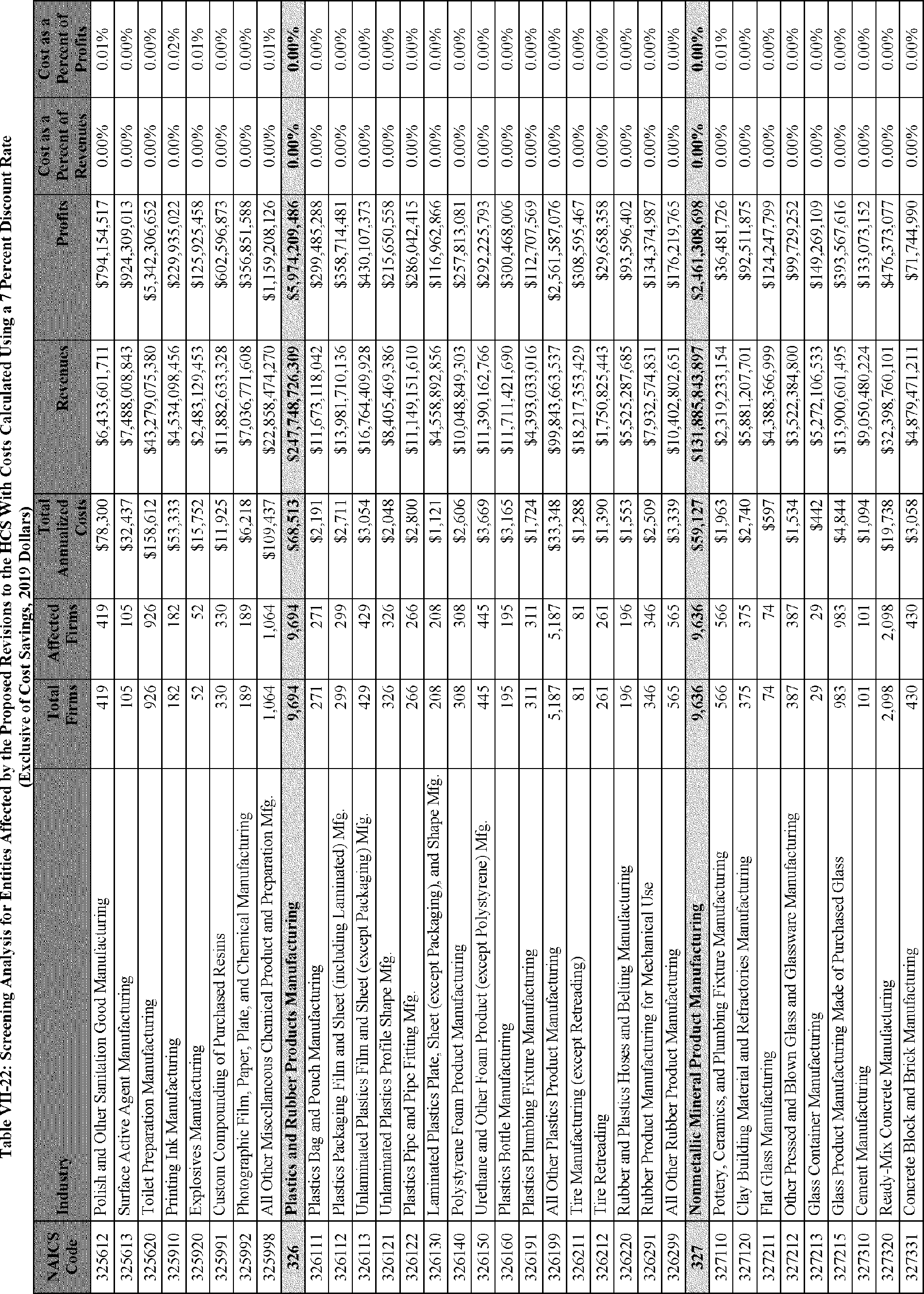
Start Printed Page 9666
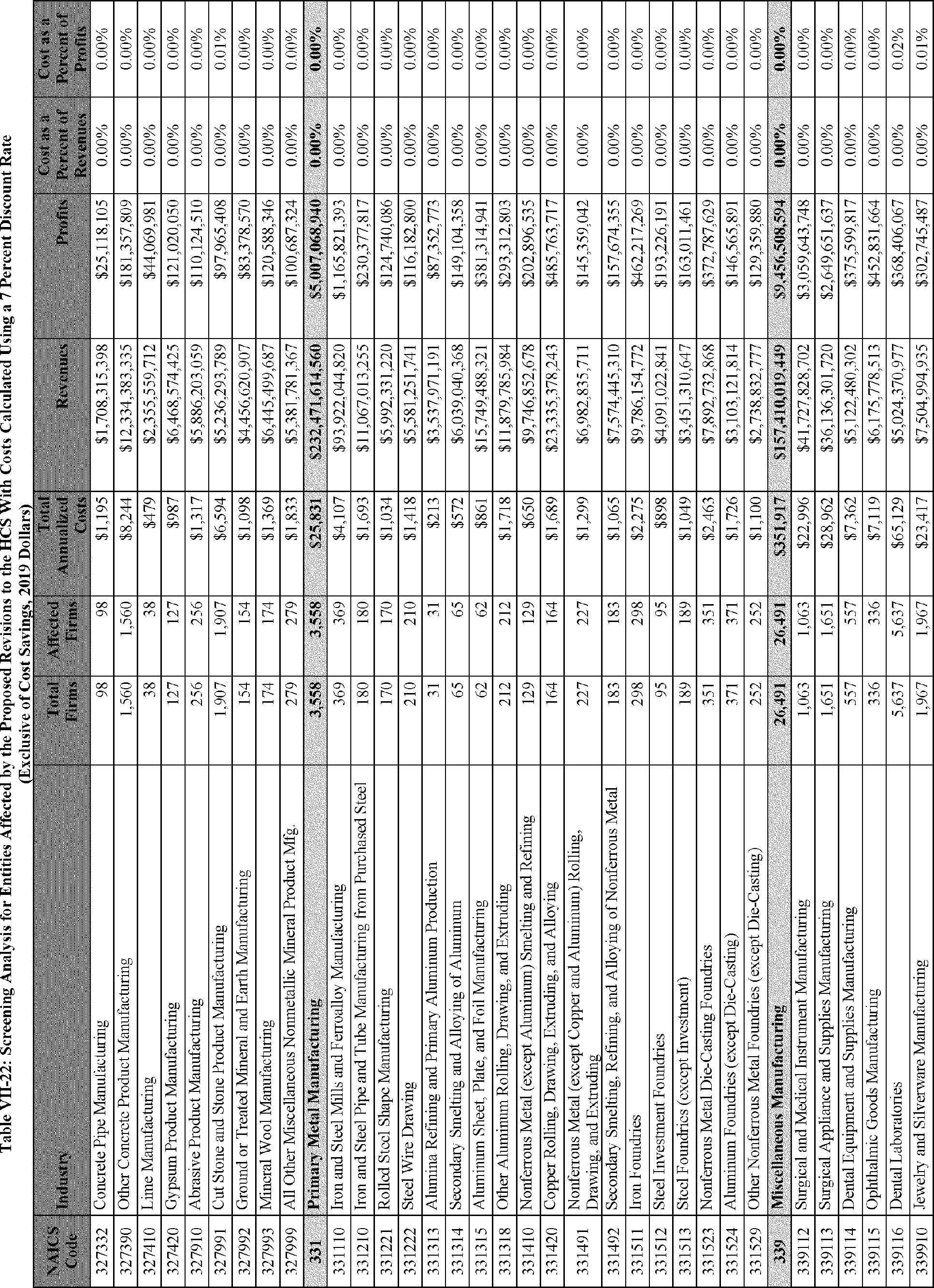
Start Printed Page 9667

In the case of costs that may be incurred due to the requirements of the proposed revisions to the HCS, all businesses within each of the covered industry sectors would be subject to the same requirements. Thus, to the extent Start Printed Page 9668 potential price increases correspond to costs associated with achieving compliance with the revised standard, the elasticity of demand for each entity will approach that faced by the industry as a whole.
Furthermore, if OSHA adopts the proposed revisions to the HCS, hazardous chemicals distributed in the United States will have to be in compliance with the updated provisions, and chemical producers and users in most advanced economies will be operating under comparable requirements based on the GHS specific to their own country or economic union. For this reason, affected domestic establishments should not be susceptible to a loss of domestic market share resulting from the competition of foreign commercial entities not bound by the requirements of the HCS or similar GHS requirements.
Given the small increases in prices potentially resulting from compliance with the proposed revisions to the HCS in any particular industry, and the lack of readily available substitutes for the products and services provided by the covered industry sectors, demand is expected to be sufficiently inelastic in each affected industry to enable entities to substantially offset compliance costs through minor price increases without experiencing any significant reduction in revenues or profits. For example, for NAICS 324191: Petroleum Lubricating Oil and Grease Manufacturing, even if zero cost savings are obtained and gross positive costs reach OSHA's estimated total ($1,221,994; see Table VII-22), revenue impacts (0.0074 percent, rounded to 0.01 percent) and profit impacts (0.108 percent, rounded to 0.11 percent) fall well below OSHA's screening criteria associated with economic feasibility concerns. OSHA therefore preliminarily concludes that the proposed rule, if implemented, would be economically feasible. The agency invites comments on this preliminary conclusion.
H. Preliminary Regulatory Flexibility Act Certification
To determine whether the proposed revisions to the HCS will have a significant economic impact on a substantial number of small entities, OSHA evaluated the impact of compliance costs on the revenues and profits of small entities in affected industries. As discussed previously, the proposed rule would impose costs on impacted industries for training; for reclassification of aerosols, desensitized explosives, and flammable gases; and for becoming familiar with the proposed changes to the standard. The proposed rule would also result in cost savings to the extent it would limit employers' duties with respect to the labeling of some very small containers and provide more flexible relabeling requirements for packaged chemicals released for shipment.
OSHA believes that the estimated costs are one-time costs that would be incurred during the first-year transition period after the rule is promulgated. In addition, as mentioned above, there will be annual cost savings due to the flexibilities introduced in the proposed provision related to the labeling of very small containers and in the proposed released-for-shipment provision.
Tables VII-23 and VII-24 present OSHA's screening analysis of the impact of compliance costs and cost savings on revenues and profits of small and very small entities. Tables VII-25 and VII-26 present OSHA's screening analysis of impacts on revenues and profits for small and very small entities under the scenario that zero-cost savings are realized, i.e., only positive costs are incurred by affected employers. OSHA's screening criteria for determining whether there are significant economic impacts on small firms assesses whether, for small entities in any given industry, the annualized costs exceed one percent of revenues or five percent of profits.[57]
The total annualized cost savings resulting from the proposed revisions to the HCS for small entities and very small entities are estimated to be approximately $17.1 million and $1.7 million, respectively (see Tables VII-23 and VII-24). To assess the potential economic impact of the proposed rule on small entities and very small entities, OSHA calculated the ratios of compliance costs to profits and to revenues. These ratios are presented for each affected industry in Tables VII-23 (small entities) and VII-24 (very small entities). Those tables show that in no industries do the annualized costs of the proposed revisions to the standard exceed one percent of annual revenues or five percent of annual profits, either for small entities or for very small entities. Similarly, under a cost scenario exclusive of cost savings (shown in Tables VII-25 and VII-26), in no industries do the annualized costs of the proposed rule exceed one percent of annual revenues or five percent of annual profits. Because no adverse revenue and profit impacts are expected to result from this proposed revision to the HCS, OSHA preliminarily certifies that the proposed changes to the standard will not have a significant economic impact on a substantial number of small entities. The agency invites comments on this preliminary certification.
Start Printed Page 9669
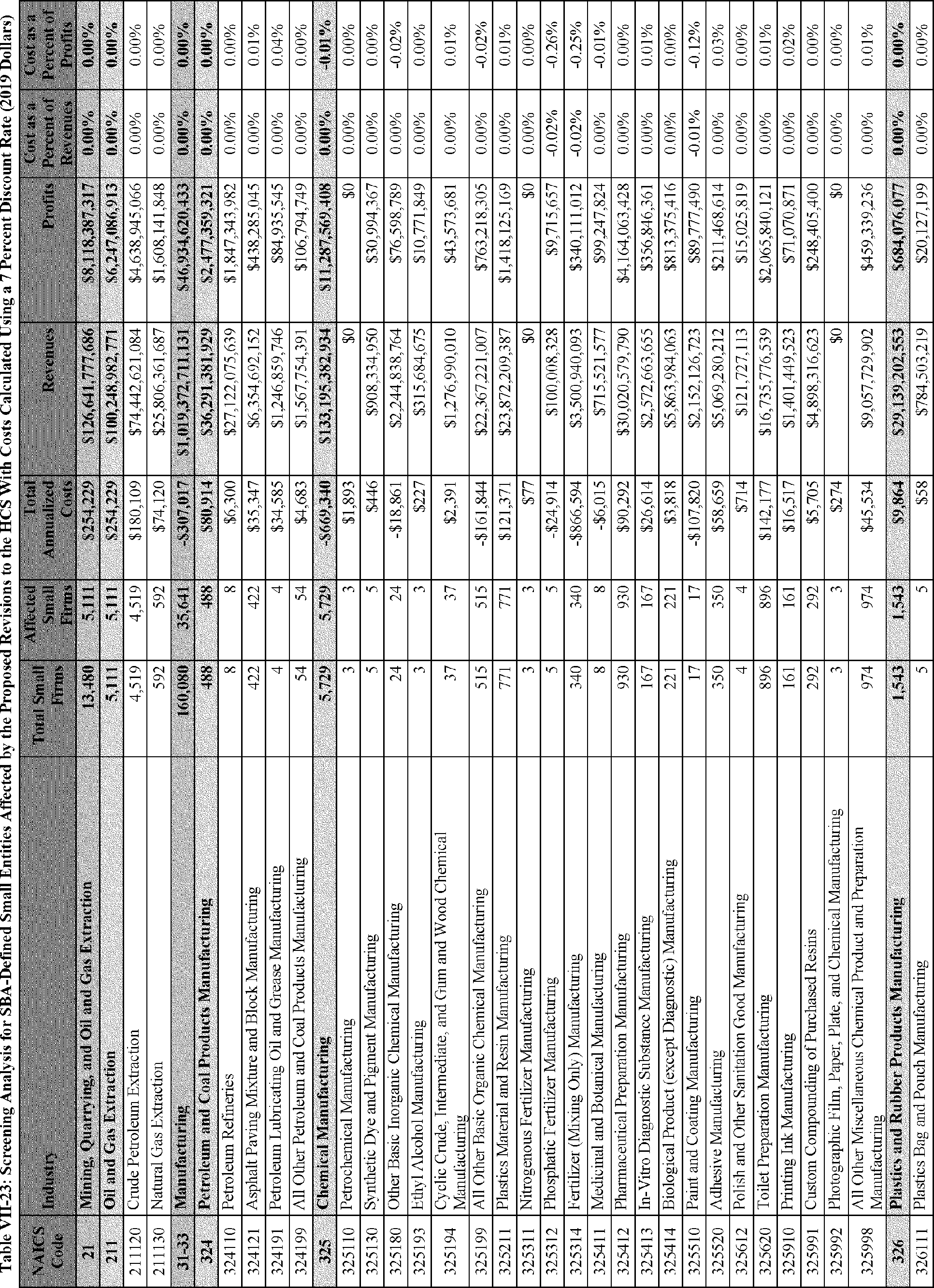
Start Printed Page 9670

Start Printed Page 9671

Start Printed Page 9672

Start Printed Page 9673

Start Printed Page 9674

Start Printed Page 9675

Start Printed Page 9676

Start Printed Page 9677

Start Printed Page 9678

Start Printed Page 9679

Start Printed Page 9680

Start Printed Page 9681

Start Printed Page 9682

Start Printed Page 9683

Start Printed Page 9684

Start Printed Page 9685

Start Printed Page 9686
VIII. Federalism
OSHA reviewed the proposed updates to the HCS according to the most recent Executive order on federalism (E.O. 13132, 64 FR 43255), which requires that Federal agencies, to the extent possible, refrain from limiting State policy options, consult with States before taking actions that would restrict States' policy options and take such actions only when clear constitutional and statutory authority exists and the problem is of national scope. The Executive order generally allows Federal agencies to preempt State law only with the expressed consent of Congress. Federal agencies must limit preemption of State law to the extent possible.
Under section 18 of the OSH Act, 29 U.S.C. 667, Congress expressly provides that States and U.S. territories may adopt, with Federal approval, a plan for the development and enforcement of occupational safety and health standards. OSHA refers to such States and territories as State Plan States. Occupational safety and health standards developed by State Plan States must be at least as effective in providing safe and healthful employment and places of employment as the Federal standards and, when applicable to products that are distributed or used in interstate commerce, must be required by compelling local conditions and not unduly burden interstate commerce. 29 U.S.C. 667(c)(2). Subject to these requirements, State Plan States are free to develop and enforce their own requirements for safety and health standards.
In States without OSHA-approved State plans, Congress expressly provides for OSHA standards to preempt State occupational safety and health standards in areas addressed by the Federal standards. In these States, the proposed revisions to the HCS would limit State policy options in the same manner as every standard or amendment to a standard promulgated by OSHA. In States with OSHA-approved State plans, the proposed revisions to the HCS would not significantly limit State policy options to adopt stricter standards.
OSHA previously concluded that promulgation of the HCS complies with E.O. 13132 (77 FR 17687), and reaffirms that finding with respect to the proposed revisions to that standard.
VIX. State Plan States
When Federal OSHA promulgates a new standard or more stringent amendment to an existing standard, the 28 States and U.S. territories with their own OSHA-approved occupational safety and health plans ("State Plan States") must amend their standards to reflect the new standard or amendment or show why such action is unnecessary, e.g., because an existing State standard covering this area is "at least as effective" as the new Federal standard or amendment. 29 CFR 1953.5(a). The State standard must be at least as effective as the final Federal rule, and, when applicable to products that are distributed or used in interstate commerce, must be required by compelling local conditions and not unduly burden interstate commerce. 29 U.S.C. 667(c)(2). State Plans must adopt the Federal standard or complete their own standard within six months of the promulgation date of the final Federal rule. When OSHA promulgates a new rule or amendment that does not impose additional or more stringent requirements than existing standards, State Plan States are not required to amend their standards, although OSHA may encourage them to do so.
The 22 States and territories with OSHA-approved occupational safety and health plans that cover public and private-sector employees are Alaska, Arizona, California, Hawaii, Indiana, Iowa, Kentucky, Maryland, Michigan, Minnesota, Nevada, New Mexico, North Carolina, Oregon, Puerto Rico, South Carolina, Tennessee, Utah, Vermont, Virginia, Washington, and Wyoming. Another six states and territories have OSHA-approved occupational safety and health plans that cover State and local government employees only: Connecticut, Illinois, Maine, New Jersey, New York, and the Virgin Islands.
X. Unfunded Mandates Reform Act
OSHA reviewed this proposal according to the Unfunded Mandates Reform Act of 1995 (UMRA), U.S.C. 1501 et seq., and Executive Order 13132 (64 FR 43255). As discussed in the Preliminary Economic Analysis, OSHA has preliminarily concluded that the proposed revisions to the HCS will not impose a Federal mandate on the private sector in excess of $100 million (adjusted annually for inflation) in expenditures in any one year.
As noted previously, OSHA's standards do not apply to State and local governments except in States that have elected voluntarily to adopt a State Plan approved by the agency. Consequently, this proposal does not meet the definition of a "Federal intergovernmental mandate." See 2 U.S.C. 658(5).
The OSH Act does not cover tribal governments in the performance of traditional governmental functions, though it generally does cover tribal governments when they engage in commercial activity. The proposed changes to the HCS would not require tribal governments to expend, in the aggregate, $100 million or more in any one year for their commercial activities.
For these reasons, for the purposes of the UMRA, OSHA certifies that this proposal would not mandate that State, local, or tribal governments adopt new, unfunded regulatory obligations of, or increase expenditures by the private sector by, more than $100 million in any year. In any event, the Preliminary Economic Analysis constitutes a written statement containing a qualitative and quantitative assessment of the anticipated costs and benefits. See 2 U.S.C. 1532.
XI. Protecting Children From Environmental Health and Safety Risks
Executive Order 13045 (62 FR 19885), requires that Federal agencies submitting covered regulatory actions to OMB's Office of Information and Regulatory Affairs (OIRA) for review pursuant to E.O. 12866 provide OIRA with (1) an evaluation of the environmental health or safety effects that the planned regulation may have on children, and (2) an explanation of why the planned regulation is preferable to other potentially effective and reasonably feasible alternatives considered by the agency. E.O. 13045 defines "covered regulatory actions" as rules that are likely to (1) be economically significant under E.O. 12866 (i.e., a rulemaking that has an annual effect on the economy of $100 million or more, or would adversely affect in a material way the economy, a sector of the economy, productivity, competition, jobs, the environment, public health or safety, or State, local, or tribal governments or communities), and (2) concern an environmental health risk or safety risk that an agency has reason to believe may disproportionately affect children. In this context, the term "environmental health risks and safety risks" means risks to health or safety that are attributable to products or substances that children are likely to come in contact with or ingest (e.g., through air, food, water, soil, or product use).
OSHA has preliminarily determined that the proposed revisions to the HCS are not economically significant under E.O. 12866 (see Section VII of this preamble) and that the standard would not pose environmental health or safety Start Printed Page 9687 risks to children as set forth in E.O. 13045.
XII. Environmental Impacts
OSHA has reviewed the proposed revisions to the HCS according to the National Environmental Policy Act of 1969 (NEPA) (42 U.S.C. 4321 et seq.), the regulations of the Council on Environmental Quality (40 CFR part 1500), and the Department of Labor's NEPA procedures (29 CFR part 11). As a result of that review, OSHA has made a preliminary determination that the proposed regulatory changes will have no impact on air, water, or soil quality; plant or animal life; or the use of land or aspects of the external environment. Therefore, OSHA preliminarily concludes that the proposed revisions to the HCS would have no significant environmental impacts.
XIII. Consultation and Coordination With Indian Tribal Governments
OSHA reviewed the proposed revisions to the HCS in accordance with E.O. 13175 on "Consultation and Coordination with Indian Tribal Governments" (65 FR 67249), and determined that it does not have "tribal implications" as defined in that order. The amendments, if promulgated, would not have substantial direct effects on one or more Indian tribes, on the relationship between the Federal Government and Indian tribes, or on the distribution of power and responsibilities between the Federal Government and Indian tribes.
XIV. Issues and Options Considered
OSHA is providing this issues and options section to solicit stakeholder input on various regulatory issues and to allow for some potential regulatory flexibility with respect to the content of any final rule resulting from this rulemaking. While OSHA invites stakeholders to comment on all aspects of this proposal, this section identifies specific areas of interest to the agency. OSHA is including certain issues and questions in this section to assist stakeholders as they review the proposal and consider the comments they plan to submit. However, to fully understand the questions, and to provide substantive input and feedback in response to them, the agency suggests commenters review the other sections of the preamble that address these issues in detail. Some issues and options that have cost implications are discussed more thoroughly in the Preliminary Economic Analysis (see Section VII. Preliminary Economic Analysis and Initial Regulatory Flexibility Analysis).
A. Issues
In this section, OSHA solicits public feedback on specific issues associated with the proposed revisions to the HCS. It should be noted that the proposed regulatory text provided at the end of this document only includes those paragraphs that OSHA is proposing to change. Therefore, the agency is putting a marked-up version (redline strike out) of the text of the current rule on its web page and in the docket to help readers identify and understand the proposed changes in context (OSHA HCS Redline, 2020, Document ID 0222). The marked-up text will be found on www.osha.gov under Hazard Communication in the subject index.
OSHA has organized this issues section to follow the order of the preamble and requests that feedback be organized, to the extent possible, in similar order. Comments and feedback on particular provisions should contain the heading of the section (e.g., Regulatory Text, Appendix A), the associated issue number, and, where appropriate, the paragraph in the standard that the comment is addressing. Comments addressing more than one section or paragraph should include all relevant references. Submitting comments in an organized manner with clear reference to the issue(s) raised will enable all participants to better understand the issues the commenter addressed and how they addressed them. Some commenters may confine their interest (and comments) to the issues that specifically affect them; correspondingly they will benefit from being able to quickly identify comments on these issues in others' submissions. While the agency welcomes relevant comments on any aspect of this proposal, OSHA is especially interested in responses, supported by evidence and explanations, to the following issues and questions:
Timeframe for Updates to the HCS
Since aligning the HCS with the GHS Rev. 3 in 2012, OSHA has intended for the HCS to stay current with more recent revisions of the GHS. The GHS is updated biennially through published revisions; most recently, revision 8 was published in July 2019 (UN GHS, Rev. 8, Document ID 0065). Regulatory authorities around the world have implemented the GHS at stages ranging from revision 1 through revision 5. Few regulatory authorities have put programs in place to update their regulations on a routine schedule. The European Union (EU) has made the most regular updates, and has most recently implemented the GHS Rev. 5 in August, 2016 (ECHA, 2016, Document ID 0177). In March 2019, the European Commission (EC) published the adaptation of technical progress (ATP) to EC regulation 1272/2008 (the Classification, Labelling, and Packaging (CLP) regulation) to align with both the sixth and seventh revised editions of the GHS (EC, 2019, Document ID 0176). These changes to the EC regulation become effective October 17, 2020. Other regulatory authorities, such as those in Canada, Australia, and New Zealand, have indicated that they will continue to update their regulations to align with the GHS and are in the process of aligning with Rev. 7; however, none of these countries have a mandate on how often they should do so (Canada, 2019, Document ID 0172; Australia, 2020, Document ID 0168; New Zealand, 2018, Document ID 0187). Similarly, to date, OSHA has not adopted a specific timeframe for regularly updating the HCS to implement GHS updates.
As stated previously, OSHA is proposing to align the HCS with the GHS Rev. 7, consistent with the actions of most of the countries listed above (EC, 2019, Document ID 0176; Canada, 2019, Document ID 0172; Australia, 2020, Document ID 0168; New Zealand, 2018, Document ID 0187). A more thorough explanation of OSHA's preliminary decision to align the HCS with the GHS Rev. 7 is detailed in the introduction to Section XV.
OSHA requests public comment on whether the agency should adopt a schedule for updates to the HCS standard (e.g., every four years or every two revisions of the GHS) or wait until there are significant changes to the GHS before initiating rulemaking. More frequently updating the HCS to align with the GHS may provide greater protection for workers and reduce uncertainty for manufacturers, distributors, and employers. For example, in the GHS Rev. 7, several hazard classes have been updated to include additional hazard sub-categories and improved hazard information that will increase clarity and, therefore, protections for workers.
OSHA is interested in receiving public comment about the utility, costs, or other issues that might be associated with regular updates and about specific timeframes or criteria that OSHA should consider when determining when and whether to update the HCS. Specifically, would longer time periods between updates and realignment with the GHS and other standards be more or less burdensome for employers, especially those that operate Start Printed Page 9688 internationally? Would regular, shorter time periods provide more stability? How would longer or shorter periods between realignment affect worker protection?
Regulatory Text
(1) Under paragraph (f), Labels and other forms of warning, OSHA is proposing changes to paragraphs (f)(5) (bulk shipments) and (f)(11) (released-for-shipment) and is also proposing to add a new paragraph (f)(12) containing provisions specific to labelling on small containers.
(a) OSHA is requesting comments on the proposed additions to paragraph (f)(5), which would be newly titled Transportation. Proposed paragraph (f)(5)(ii) would provide that labels for bulk shipments may be placed on the immediate container or may be transmitted with the shipping papers or bills of lading or by other technological or electronic means as long as the label is immediately available to workers in printed form at the receiving end of the shipment. OSHA requests comments on the usefulness and effectiveness of allowing these alternate approaches for labeling bulk shipments.
(b) OSHA is proposing to update paragraph (f)(11) to provide that chemicals that have been released for shipment and are awaiting future distribution need not be relabeled to incorporate new significant information about hazards; however, the chemical manufacturer or importer would still have to provide the updated label for each individual container with each shipment. The purpose of this proposal is to account for the long distribution cycles of some products and the potential hazards workers could face in relabeling the immediate containers of hazardous chemicals (e.g., chemical exposures, ergonomic issues). OSHA requests comments on whether it is appropriate to use "released for shipment" as the cutoff point for relabeling requirements, as opposed to, for example, the time of shipment. Would the proposed provision reduce worker protections, considering OSHA is also proposing to require that the updated label be sent with the shipment? Would the proposed change result in any cost savings?
(c) OSHA is proposing a new paragraph (f)(12) addressing labeling requirements for small containers. All of the provisions in this proposed paragraph would apply only where the chemical manufacturer, importer, or distributor can demonstrate that it is not feasible to use pull-out labels, fold-back labels, or tags containing the full label information required by paragraph (f)(1). Paragraph (f)(12)(ii), as proposed, would provide that labels for small containers less than or equal to 100 ml capacity must include just the product identifier, pictogram(s), signal word, chemical manufacturer's name and phone number, and a statement that the full label information for the hazardous chemical is provided on the immediate outer package. In addition, proposed (f)(12)(iii) would eliminate labeling requirements for small containers less than or equal to 3 ml capacity where the manufacturer, importer, or distributor can demonstrate that any label would interfere with the normal use of the container; in such cases, however, the proposed revisions to the standard would require the container to bear, at a minimum, the product identifier. For example, the product identifier could be etched on a small glass vial. This would ensure that each small container can be identified and linked with the full label information on the immediate outer package. OSHA is also proposing a provision at paragraph (f)(12)(iv), applicable to all small containers covered by paragraph (f)(12)(ii) or (iii), providing that the immediate outer package must include (1) the full label information for each hazardous chemical in the immediate outer package; and (2) a statement indicating that the small container(s) inside must be stored in the immediate outer package (bearing the complete label) when not in use. OSHA requests comments on the feasibility of, and any cost savings associated with, these proposed provisions for the labeling of small containers (both 100 ml and less and 3 ml and less). The agency also requests information on whether the proposed labeling requirements would be adequate to provide for safe handling and storage of chemicals in small containers. In addition, OSHA is interested in receiving comments on two specific alternatives to proposed paragraph (f)(12). First, instead of adopting proposed paragraph (f)(12), should OSHA simply allow for case-by-case exemptions if full labeling is not feasible? Second, should the agency require a showing that a full label would interfere with the normal use of the container before permitting the use of abbreviated labels on containers with a capacity of 100 ml and less (similar to the condition OSHA is proposing in paragraph (f)(12)(iii) for containers with a capacity of 3 ml and less)? Please provide reasons for your answers.
(2) Under paragraph (g) Safety data sheets, OSHA is proposing a change to paragraph (g)(10), which addresses the form and storage of safety data sheets, to allow SDSs to be stored, rather than designed, in a way that covers groups of hazardous chemicals in a work area. The original term "design" was used when OSHA did not require a specific format for material safety data sheets (MSDSs), but now that OSHA requires SDSs to be in a standard 16-section format, the agency is proposing to clarify that this paragraph refers to storage only. OSHA requests comments regarding whether this proposed revision would require significant changes to current practices.
(3) Under paragraph (i), Trade secrets, OSHA is proposing two significant changes.
(a) First, OSHA is proposing to allow manufacturers, importers, and employers to withhold a chemical's concentration range as a trade secret.
(b) Second, in proposed paragraphs (i)(1)(iv)(A) through (M), OSHA is proposing the use of prescriptive concentration ranges in lieu of the actual concentration or concentration range whenever the actual concentration or concentration range is claimed as a trade secret; the proposed ranges are the same as those required by Canada, a major trading partner of the United States (Canada, 2019, Document ID 0172).
OSHA currently does not permit manufacturers to claim concentration ranges as trade secrets (Colau, 2017, Document ID 0098; Nelson, 2017, Document ID 0099), and is requesting comments on its proposal to do so. Specifically, the agency is interested in any experience stakeholders have had with developing SDSs using the prescribed concentration ranges and any concerns stakeholders have about using concentration ranges on the SDS. The agency is also requesting comments addressing the adequacy of hazard information provided by these ranges. Do these ranges provide sufficient information for downstream manufacturers to conduct hazard classifications? Are the ranges prescribed too wide to provide sufficient information to protect workers (i.e., should they be narrowed)? Notably, proposed paragraph (i)(1)(v) provides that the prescribed concentration range used must be the narrowest range possible. If the exact concentration range falls between 0.1% and 30% (proposed paragraphs (i)(1)(iv)(A) through (G)) and does not fit entirely into one of the prescribed concentration ranges, a single range created by the combination of two applicable consecutive ranges could be disclosed instead, provided that the combined concentration range does not include any range that falls entirely outside the Start Printed Page 9689 exact concentration range in which the ingredient is present. OSHA invites comments on whether it should allow combinations among all ranges (i.e., all of the ranges (up to 100% concentration) listed in proposed paragraphs (i)(1)(iv)(A) through (M)) or whether the rule applicable to combining ranges should be even more restrictive (e.g., only for the ranges (up to 10% concentration) listed in proposed paragraphs (i)(1)(iv)(A) through (E)). OSHA is also interested in receiving comments on whether there are any economic implications associated with including the prescribed concentration ranges.
Appendix B
OSHA is proposing several substantive updates to appendix B (as outlined in Section XV, Summary and Explanation). These include the addition of a new hazard class (desensitized explosives) and several new hazard categories (unstable gases and pyrophoric gases in the Flammable Gases class and nonflammable aerosols in the Aerosols class). OSHA has preliminarily determined that the addition of these specific hazard classes and categories would better differentiate between the hazards and better communicate hazards on labels for downstream users. OSHA is requesting comments on whether these changes provide improved safety through more targeted hazard statements, precautionary statements and pictograms.
Appendix C
OSHA has proposed numerous changes to appendix C, many of which are editorial, clarifying, or organizational in nature and are designed to clarify requirements for preparing labels. The agency is also proposing some substantive changes to correspond to proposed changes to appendix B or the regulatory text. In paragraph C.2.4.10, OSHA is proposing to require prioritization of certain precautionary statements related to medical response (see Section XV, Summary and Explanation, Appendix C, Proposed Revisions to Table C.2.4.). The agency requests comments on the particular system of prioritization specified in proposed C.2.4.10 and on whether the proposed prioritization provisions would improve clarity on labels.
Appendix D
Many of the issues related to changes proposed for appendix D are discussed in the summary and explanation of the regulatory text (see Section XV, Summary and Explanation, Regulatory Text), specifically in the discussion of OSHA's proposed changes to paragraphs (c), (g), and (i). OSHA requests comments on the following additional issues:
OSHA is proposing changes to section 2 of the SDS to emphasize that hazards identified under normal conditions of use that result from a chemical reaction must appear on the SDS, even though these hazards do not need to be listed on the label. This proposed change would simply reorganize the information presented in the SDS, as discussed in Section XV (Summary and Explanation, Appendix D). OSHA is requesting comments on whether the text OSHA is proposing for paragraph (c) in section 2 would clarify when it is appropriate to include information on the hazards associated with a change in the chemical's physical form or chemical reaction under normal conditions of use and the type of information that should be presented in section 2 of the SDS.
With some conditions, the HCS currently requires section 3 of the SDS to include the chemical name and concentration (exact percentage) or concentration ranges of all ingredients which are classified as "health hazards" in accordance with paragraph (d) of § 1910.1200. OSHA is not proposing to change this requirement, but is interested in comments on whether it should be expanded to include all classified chemicals (i.e., also physical hazards and HNOCSs). Such a requirement would be similar to the EU REACh regulations, which require SDS preparers to list the classification of each hazardous ingredient (ECHA, 2016, Document ID 0177). Would expanding the requirements for section 3 in this way ensure that both users and manufacturers fully understand any potential hazard when handling the chemical? Would such a change result in the provision of additional information that would allow downstream manufacturers to more accurately classify their products where the mixture in question is one of their ingredients?
The use of newer electronic technology, such as quick response (QR) codes and radio-frequency identification (RFID), on package labels give responsible parties the ability to communicate information on chemical hazards in a variety of formats. In the December 2018 session of the UN Sub-committee, the members of an informal working group on labeling of small containers agreed to extend its scope beyond small containers and, accordingly, to change its name to "Practical Labelling Issues." Among other activities proposed for the biennium 2019-2020, the working group planned to "[r]eview the existing digital means of communication that can be used to convey the GHS hazard information to users (e.g., electronic label, QR code etc.)," "consider the development of general principles and criteria on the provision of this information digitally," and "develop guidance and examples wherever appropriate." (UN GHS, 2019, Document ID 0198; UN Secretariat, 2019, Document ID 0196).
As an example, a paper presented at the December 2018 session of the UN sub-committee noted that there are international efforts "actively promoting the application of electronic labels for chemicals" in such industrial processes as production; management of cylinders, laboratory samples, and warehouse operations; and the supervision of competent persons (UN GHS, 2019, Document ID 0198). The paper noted that common types of electronic labels include QR codes and RFID. The paper also discussed efforts to develop national standards on electronic labeling "to establish a complete integrated information managing standard system based on chemical electronic labels and safety data" in order to "further ensure the effectiveness of chemical safety supervision, promote the implementation of the Globally Harmonized System of Classification and Labelling of Chemicals, and facilitate . . . trade." Among the benefits of practical labeling cited by the sub-committee expert are the convenience and efficiency derived from "unified information collection," "dynamic management," and "real-time monitoring"; the ability to store a large capacity of information, reaching multiple mega-byte levels; and "[improvement in] the level of safety management in complex scenarios." (UN GHS, 2018, Document ID 0082).
OSHA invites comments on the use of electronic labeling for chemical packaging. If a future revision to the HCS permitted some form of electronic labeling, what technological, economic, and security challenges would affected employers face? The agency also requests comments on the types of electronic chemical labeling already in existence or under development. For employers already implementing electronic labeling programs in the United States or in other countries, please provide information on the types of electronic coding systems utilized in the program and the costs incurred and benefits achieved from the program. Start Printed Page 9690 What back-up measures are in place to ensure immediate access to the hazard information? OSHA is interested in information about workers' experiences with the use of electronic labels. OSHA also requests comments on foreseeable challenges that OSHA should consider (e.g., worker accessibility to electronic label information).
Preliminary Economic Analysis
(1) As explained in the preliminary economic analysis (see Section VII.F. Compliance Costs and Cost Savings), some chemical production and logistics employees who receive training under the provisions of the existing HCS would need to receive additional training to become familiar with the updates to SDSs and labels for impacted aerosols, desensitized explosives, and flammable gases. OSHA has preliminarily determined that the incremental training costs employers would incur for these employees will be relatively small (estimated annualized training costs of $120,158 for all affected employers). OSHA also believes that users of aerosols, desensitized explosives, and flammable gases in the workplace are already trained on the hazards of these chemicals and therefore would need to devote only a trivial amount of time, if any time at all, to additional training associated with the reclassification of these chemicals. Per the discussion of this issue in the preliminary economic analysis (see "Costs Associated with Training Employees" in Section VII.F. Compliance Costs and Cost Savings), OSHA acknowledges that some user training might be required for non-flammable aerosols not under pressure; the agency has preliminarily concluded, however, that such training time would be negligible given that, as discussed in Section XV.C (see Section XV, Summary and Explanation for Appendix B), most aerosols are currently classified as gases under pressure and therefore are already covered under the HCS. The agency requests comments on all of its preliminary conclusions regarding training time for users of reclassified chemicals.
(2) For purposes of estimating the costs associated with the proposed new hazard classification requirements, OSHA estimates that a Health & Safety Specialist would spend 1.75 hours per SDS for establishments with fewer than 100 employees; 1.25 hours per SDS for establishments with 100-499 employees; and 0.75 hours per SDS for establishments with 500 or more employees (see "Costs Associated with Reclassifications and Revisions to Safety Data Sheets and Labels" in Section VII.F, Compliance Costs and Cost Savings). At a loaded hourly wage of $56.87, this results in estimated unit costs of $101.51, $72.51, and $43.50 per SDS for small, medium, and large establishments, respectively. OSHA invites interested parties to comment on these estimates.
(3) For purposes of estimating the costs associated with revising labels and SDSs to conform to the revisions OSHA is proposing to mandatory language in the appendices, OSHA estimates that a Health & Safety Specialist would spend 0.7 hours per SDS for establishments with fewer than 100 employees; 0.5 hours per SDS for establishments with 100-499 employees; and 0.3 hours per SDS for establishments with 500 or more employees (see "Revisions to SDSs and Labels Due to Revised Precautionary Statements," in Section VII.F. Compliance Costs and Cost Savings). At a loaded hourly wage of $56.39, this results in estimated unit costs of $40.60, $29.00, and $17.40 per SDS for small, medium, and large establishments, respectively. OSHA invites interested parties to comment on these estimates.
(4) To estimate the costs (cost savings) associated with the proposed released-for-shipment provisions in paragraph (f)(11), OSHA presented a cost methodology that required estimating four factors: (1) Cost savings (estimated relabeling costs) as a percentage of the value of the products needing relabeling; (2) the percentage of products in the affected NAICS industries that would be warehoused for more than six months; (3) the percentage of products warehoused for more than six months that would require relabeling in any particular year due to a manufacturer-initiated labeling change;[58] and (4) the percentage of all products in the NAICS industries that would be covered by the proposed revisions to the HCS. The estimated percentages are shown in Table VI-17: Calculation of the Percentage Loss Avoided Due to the Proposed Released-for-Shipment Provision. OSHA requests public comments on its estimates for each of the four factors described above and shown in Table VI-17.
(5) As described in the PEA (see "Released for Shipment" in Section VII.F. Compliance Costs and Cost Savings), OSHA anticipates that the proposed modifications to paragraph (f)(11) addressing chemicals that have been released for shipment would result in cost savings for manufacturers and distributors of certain products—those with large (and typically infrequent) production runs and lengthy shelf lives (often five years or longer) that, during production, are labeled, boxed, palletized, and shipped, and then go through the distribution chain usually without the chemical contents, packaging, or label being disturbed. OSHA identified six industries (NAICS 325311 Nitrogenous fertilizer manufacturing, NAICS 325312 Phosphatic fertilizer manufacturing, NAICS 325314 Fertilizer (mixing only) manufacturing, NAICS 325510 Paint and coating manufacturing, NAICS 424910 Farm supplies merchant wholesalers, and NAICS 424950 Paint, varnish, and supplies merchant wholesalers) that it expects would be impacted by the proposed modifications to paragraph (f)(11); see Table VI-17 in Section VII.D. Health and Safety Benefits and Unquantified Positive Economic Effects. OSHA invites comments on whether other industries would be affected by this proposed modification and whether there might be other cost or health effects resulting from this proposed modification that OSHA did not consider in this proposal.
(6) Also with respect to the estimate of cost savings associated with the proposed released-for-shipment provisions, OSHA assumes that if the relabeling costs associated with paragraph (f)(11) exceed the value of the product, manufacturers and wholesalers will discard the product rather than pay to relabel it. There may be some disposal costs for the discarded material, but there may also be some salvage value to the improperly-labeled product. In the preliminary economic analysis (see "Released for Shipment" in Section VII.F. Compliance Costs and Cost Savings), OSHA estimates, without further information on the distribution of the costs, that the average labeling cost is approximately 50 percent of the value of the products requiring relabeling. The agency invites comments on this assumption.
B. Options
In this section, OSHA presents a list of options that are under consideration for the proposed update to the HCS. The agency is requesting public comment on these options.
Regulatory Text
(1) OSHA is proposing, in paragraph (i), to mandate the use of prescriptive concentration ranges whenever an actual concentration or concentration range is being claimed as a trade secret. This change is being proposed, in part, to better align with Canada's Workplace Start Printed Page 9691 Hazardous Materials Information System (WHMIS), allowing manufacturers, importers, and employers the ability to use the same SDS for both U.S. and Canadian workplaces. However, the agency is also considering a non-mandatory option for this provision. Under this scenario, OSHA would provide non-mandatory guidance on the use of concentration ranges, but would not require their use. This would allow manufacturers, importers, and employers flexibility to follow the current HCS requirements (which do not require the use of any concentration ranges when the actual concentration is claimed as a trade secret) or move to a system that aligns with WHMIS. OSHA is requesting comments on this option. Would this option provide beneficial flexibility to manufacturers, importers, and employers? Would this option be too confusing, and potentially weaken protective effects that would be associated with providing prescribed concentration ranges? How would this affect employee safety and comprehension?
(2) Under paragraph (i), OSHA is also considering allowing manufacturers and importers to provide their own ranges as long as the range is narrower than any prescribed range. This alternative could allow manufacturers and importers to provide downstream users with more precise information while still being able to claim a trade secret. This would be consistent with an approach Health Canada is considering (Canada, 2019, Document ID 0172). OSHA is seeking comments on the usefulness and viability of this option.
Revision 8 Changes
The GHS Rev. 8 was published in July 2019 and contains many changes from Rev. 7, including updates to certain hazard classification criteria, systematic updates to the definitions in the health hazard chapters, updates to hazard and precautionary statements, and updated labeling examples. An overview of the changes can be found in Document ID 0243. As discussed more thoroughly in the introduction to the Summary and Explanation (see Section XV), OSHA has preliminarily decided to use this proposed update to align the HCS with the GHS Rev. 7. However, OSHA has also identified specific updates found in the GHS Rev. 8 that are significant enough to warrant consideration in this rulemaking. Below, the agency highlights several updates from the GHS Rev. 8 and invites public comments on whether OSHA should consider adopting these updates.
1. Appendix A (Based on the GHS Rev. 8)
OSHA is proposing substantial revisions to appendix A.2 (skin corrosion/irritation) that reflect changes the UN subcommittee adopted through the GHS Rev. 7. However, the GHS Rev. 8, published in July 2019 (UN GHS, 2019, Document ID 0065), expanded the use of non-animal test methods in Chapter 3.2 (skin corrosion/irritation). These changes include recognition of specific in vitro test methods, reorganization of the chapter, reorganization of the tiered approach with an updated Figure 3.2.1 to reflect those changes, as well as descriptive text on use of new test methods, structure activity relationship (SAR) and read across methods, and an updated decision logic diagram. The expansion of non-animal test methods for use in hazard classification could potentially result in cost savings, as hazard testing for new chemicals could be done using potentially cheaper (non-animal) test methods. If OSHA were to adopt these changes, they would be reflected in appendix A.2 Skin Corrosion/Irritation.
Start Printed Page 9692
Table 3.2.1 from the GHS Rev. 8 (shown above) provides an update to the tiered approach for classification. In recognition of the advancements made in non-animal test methods, the update includes an elevation in acceptance of in vitro data to tier 2 of the approach. The updated tiered approach also includes consideration of conflicting lower-tiered data when the lower tier suggests a higher classification level. In addition to the changes in the table, Rev. 8 updates the background information to provide additional guidance for how to use non-animal test data to classify chemicals. Adopting these updates in the HCS would not require a re-evaluation of chemicals already classified because the overall tiered approach for evaluating existing data has been retained. The agency believes the greatest benefit would be for new chemicals where no existing data currently exists. Although OSHA does not require testing, OSHA currently encourages chemical manufacturers wanting to develop hazard information for new chemicals to utilize non-animal testing strategies to develop hazard information. Should OSHA adopt Chapter 3.2 from the GHS Rev. 8 with all of the revisions to the classification scheme? Please explain your opinion and provide any relevant data or other information.
2. Appendix B (Based on the GHS Rev. 8)
In this NPRM, OSHA is proposing updates to the classification and labeling of aerosols that will align with the GHS Rev. 7. However, the GHS Rev. 8 contains several significant additional changes in the aerosol chapter. OSHA requests comments on whether the agency should adopt two specific changes that appear in the GHS Rev. 8. First, the GHS Rev. 8 lists classification criteria for aerosols as text in a table (see the GHS table 2.3.1, Criteria for aerosols), similar to other hazard chapters, rather than referring classifiers to the decision logics. When OSHA revised the HCS in 2012, the agency declined to adopt the GHS decision logics and used its own text for classification of flammable aerosols (§ 1910.1200, appendix B). OSHA has preliminarily determined that there are no substantive differences between OSHA's current text and the text Start Printed Page 9693 represented in the new Rev. 8 table (reproduced below), although they do not contain exactly the same language (UN GHS, Rev. 8, Document ID 0065).
| Category | Criteria |
|---|---|
| 1 | (1) Any aerosol that contains ≥85% flammable components (by mass) and has a heat of combustion of ≥30 kJ/g; |
| (2) Any aerosol that dispenses a spray that, in the ignition distance test, has an ignition distance of ≥75 cm; or | |
| (3) Any aerosol that dispenses a foam that, in the foam flammability test, has: | |
| (a) a flame height of ≥20 cm and a flame duration of ≥2 s; or | |
| (b) a flame height of ≥4 cm and a flame duration of ≥7 s. | |
| 2 | (1) Any aerosol that dispenses a spray that, based on the results of the ignition distance test, does not meet the criteria for Category 1, and which has: |
| (a) A heat of combustion of ≥20 kJ/g; | |
| (b) a heat of combustion of <20 kJ/g along with an ignition distance of ≥15 cm; or | |
| (c) a heat of combustion of <20 kJ/g and an ignition distance of <15 cm along with either, in the enclosed space ignition test: | |
| A time equivalent of ≤300 s/m3; or | |
| a deflagration density of ≤300 g/m3; or | |
| (2) Any aerosol that dispenses a foam that, based on the results of the aerosol foam flammability test, does not meet the criteria for Category 1, and which has a flame height of ≥4 cm and a flame duration of ≥2 s. | |
| 3 | (1) Any aerosol that contains ≤1% flammable components (by mass) and that has a heat of combustion <20 kJ/g; or |
| (2) Any aerosol that contains >1% (by mass) flammable components or which has a heat of combustion of ≥20 kJ/g but which, based on the results of the ignition distance test, the enclosed space ignition test or the aerosol foam flammability test, does not meet the criteria for Category 1 or Category 2. |
Should OSHA adopt the classification criteria for the aerosols hazard class as presented above? While the criteria themselves would not change as compared to OSHA's existing standard, adopting the precise language in the GHS text may minimize confusion.
Second, in Rev. 8, the GHS adopted a new hazard category within the aerosols class: Chemicals under pressure (UN GHS, 2019, Document ID 0065; UN GHS, 2018, Document ID 0247; UN GHS, 2018, Document ID 0248). These products function similarly to aerosol dispensers (UN 1950), but are packed in pressure receptacles (refillable and non-refillable) up to 450 liters ((UN GHS, 2019, Document ID 0065; UN TDG, 2020, Document ID 0195). Chemicals under pressure used for spray applications present hazards that are similar to those presented by aerosol dispensers. Therefore, the classification criteria and hazard information are the same as for aerosols. OSHA recognizes that adopting this hazard classification would bring some chemicals under the purview of the HCS that currently are not covered (e.g., certain aerosols in refillable containers). Should OSHA consider adopting the new hazard category of chemicals under pressure in the aerosol chapter?
3. Appendix C (Based on the GHS Rev. 8)
In this NPRM, OSHA is proposing to update a number of precautionary statements to align with the GHS Rev. 7. However, the GHS Rev. 8 includes additional revisions to precautionary statements, most notably an overhaul of the medical response precautionary statements (UN GHS, 2019, Document ID 0065). These precautionary statements were revised for the GHS Rev. 8 because, among other reasons, manufacturers and suppliers had difficulty choosing the appropriate wording where options were given (e.g., choosing between calling a poison center or doctor, or choosing between medical advice or attention) (UN GHS, 2019, Document ID 0065).
| Code | Response precautionary statements | Hazard class | Hazard category | Conditions for use |
|---|---|---|---|---|
| P316 | Get emergency medical help immediately | Acute toxicity, oral (chapter 3.1) Acute toxicity, dermal (chapter 3.1) Acute toxicity, inhalation (chapter 3.1) Skin corrosion (chapter 3.2) | 1, 2, 3 1, 2, 3. 1, 2, 3. 1, 1A, 1B, 1C. | Competent Authority or manufacturer/supplier may add, `Call' followed by the appropriate emergency telephone number, or the appropriate emergency medical help provider, for example, a Poison Centre, Emergency Centre or Doctor. |
| Respiratory sensitization (chapter 3.4) | 1, 1A, 1B | |||
| Specific target organ toxicity, single exposure; (chapter 3.8) | 1, 2 | |||
| Aspiration hazard (chapter 3.10) | 1, 2 | |||
| P317 | Get medical help | Gases under pressure (chapter 2.5) | Refrigerated liquefied gas | |
| Acute toxicity, oral (chapter 3.1) | 4, 5 | |||
| Acute toxicity, dermal (chapter 3.1) | 4, 5 | |||
| Acute toxicity, inhalation (chapter 3.1) | 4, 5 | |||
| Skin irritation (chapter 3.2) | 2, 3 | |||
| Serious eye damage (chapter 3.3) | 1 | |||
| Eye irritation (chapter 3.3) | 2/2A, 2B | |||
| Skin sensitization (chapter 3.4) | 1, 1A, 1B | |||
| P318 | If exposed or concerned, get medical advice | Germ cell mutagenicity (chapter 3.5) | 1, 1A, 1B, 2 | |
| Carcinogenicity (chapter 3.6) | 1, 1A, 1B, 2 | |||
| Start Printed Page 9694 | ||||
| Reproductive toxicity (chapter 3.7) | 1, 1A, 1B, 2 | |||
| Reproductive toxicity, effects on or via lactation (chapter 3.7) | Additional category | |||
| P319 | Get medical help if you feel unwell | Specific target organ toxicity, single exposure; respiratory tract irritation (chapter 3.8) | 3 | |
| Specific target organ toxicity, single exposure; narcotic effects (chapter 3.8) | 3 | |||
| Specific target organ toxicity, repeated exposure (chapter 3.9) | 1, 2 | |||
As the new statements used in the GHS Rev. 8 provide standardized language and do not require manufacturers and suppliers to decide which statement is most appropriate, adopting these statements in the HCS as part of this rulemaking might save manufacturers or importers time and/or money compared to the existing statements. OSHA also believes that these statements could improve hazard communication and worker safety by more effectively conveying the type of medical action that is necessary. OSHA seeks comments on the potential benefits or drawbacks associated with adopting these revised medical response statements, or other precautionary statements that are part of the GHS Rev. 8, as a part of this rulemaking (see also Summary and Explanation, Section XV.D. Appendix C). OSHA's existing enforcement policy, as described in the OSHA hazard communication directive (OSHA, 2015, Document ID 0007), addresses situations in which employers may use precautionary statements from a more recent version of the GHS; does the policy described in the directive provide sufficient flexibility?
Incorporation by Reference
OSHA is proposing to revise the general incorporation by reference section, 29 CFR 1910.6, to include updated test methods referenced in the proposed revisions to the HCS. OSHA does not intend to require chemicals already classified using an earlier version of a consensus standard to be reclassified. OSHA believes that requiring the reclassification of chemicals based on updated test methods could result in unnecessary economic impacts and create unnecessary confusion for stakeholders. OSHA is considering ways to clarify this in the final regulatory text, e.g., by including a provision in the Dates section of the rule stating that chemicals classified based on older test methods, prior to the effective date of the rule, do not need to be reclassified, and invites comments on this topic.
XV. Summary and Explanation of the Proposed Modifications to the Hazard Communication Standard
This section of the preamble explains OSHA's proposed changes to the HCS (29 CFR 1910.1200). OSHA is proposing to align this modification of the HCS with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060). The agency has preliminarily decided to base most of the GHS alignment on Rev. 7 for several reasons, even though Rev. 8 of the GHS was issued in July 2019 (UN GHS, 2019, Document ID 0065). First, OSHA began its work to update the standard prior to the release of Rev. 8. While OSHA has always intended the HCS to be evergreen, preparation for amending any standard is a time-consuming process and changing course would have resulted in a significant delay to this rulemaking. Second, the U.S.'s major trading partners (Canada, Europe, Australia, and New Zealand) are all preparing to align with Rev. 7 (Canada, 2019, Document ID 0172; Australia, 2020, Document ID 0168; New Zealand, 2018, Document ID 0187; EC, 2019, Document ID 0176). Third, OSHA has discussed the potential for adopting some of the most consequential changes from Rev. 8 in the Issues and Options section (see Section XIV, Issues and Options Considered).
In general, OSHA has received broad support for this rulemaking. During OSHA's informal discussion with stakeholders in November 2016 about the potential changes to the HCS (Docket No. OSHA-2016-005), the agency received feedback supporting continued alignment of the HCS with the GHS and Health Canada, as well as support for addressing various implementation issues under the existing HCS (API, 2016, Document ID OSHA-2016-0005-0026; ACC, 2016, Document ID OSHA-2016-0005-0014; NGFA, 2016, Document ID OSHA-2016-0005-0018; AFIA, 2016, Document ID OSHA-2016-0005-0017). The proposed changes are intended to improve and enhance worker protection with regard to hazard communication by incorporating new hazard classes and categories, improving and streamlining precautionary statements, and providing additional clarification of existing regulatory requirements. The following is a discussion, by provision, of the proposed revisions to the standard.
In the discussion of the proposed modifications to the appendices, OSHA describes certain proposed changes that would affect multiple hazard classes. OSHA discusses some changes in general terms and indicates where those changes occur. However, to aid stakeholders, so they can see the proposed changes in context, OSHA is placing in the docket and on its website a redline strikeout version of all of the proposed revisions to the current HCS and appendices (OSHA HCS Redline, 2020, Document ID 0222; https://www.osha.gov/dsg/hazcom/).
A. Incorporation by Reference
OSHA is proposing to update the general incorporation by reference section, 29 CFR 1910.6, to include the national/international consensus standards listed below. OSHA does not intend to require chemicals already classified using an earlier version of a consensus standard to be reclassified. OSHA believes that requiring the reclassification of chemicals based on updated test methods could result in unnecessary economic impacts and create unnecessary confusion for stakeholders. OSHA is considering ways to clarify this in the final regulatory text, e.g., by including a provision in the DATES section of the rule stating that chemicals classified based on older test methods, prior to the effective date of the rule, do not need to be reclassified, and invites comments on this topic.
In places where OSHA is proposing to cite to new or updated national/international consensus standards in the regulatory text and appendix B, OSHA Start Printed Page 9695 is proposing to include the title, edition/version, and year of the standard in the relevant reference for the benefit of stakeholders and for ease of compliance. OSHA is not currently proposing to add/update all existing references to consensus standards in the regulatory text and appendix B, but recognizes that in some places in the existing text consensus standards are cited without specific reference to the year, edition, or full title of the relevant standard. In such cases, stakeholders need to consult with § 1910.6 to find more details regarding the specific consensus standard that has been incorporated by reference in the HCS. For example, appendix B, Section B.6.3 (Flammable Liquids), incorporates by reference ASTM D1078, and § 1910.6 specifies that the version of that standard incorporated by reference is the one approved on May 15, 2005. Since there are many versions of ASTM D1078 available, OSHA realizes that the general reference to ASTM D1078 in appendix B could cause confusion to those classifying new chemicals. OSHA is requesting comments on whether additional information (year, edition/version, full title) should be added to all of the references to consensus standards that are already incorporated by reference in the HCS.
OSHA is proposing to incorporate by reference (in § 1910.6) the materials below. A brief description of each consensus standard is provided in the text below. A description of their use can be found in the Regulatory Text, Appendices, and Summary and Explanation for the Regulatory Text and Appendices (see Section XV.A and D) where the standard is referenced. Each standard is available for purchase through the publication agencies listed below:
• Regulatory Text—Paragraph c (Definitions)
○ ASTM D 4359-90 (2019)—Standard Test Method for Determining Whether a Material is a Liquid or a Solid, Re-approved 2019: This consensus standard provides specific details regarding the test methods used to determine whether a viscous material is a liquid or solid.
ASTM, International: https://astm.org/Standard/standards-and-publications.html.
○ European Agreement Concerning the International Carriage of Dangerous Goods by Road (ADR): This consensus standard provides test methods for determining the specific physical characteristics of a liquid.
https://shop.un.org/series/european-agreement-concerning-international-carriage-dangerous-goods-road-adr.
• Appendix B.1.3—Explosives
○ UN ST/SG/AC.10/30/Rev.6, UN Recommendations on the Transport of Dangerous Goods, Manual of Tests and Criteria, Part I: This consensus standard provides test methods to determine if a substance has explosive properties, the degree of sensitivity of the explosive properties, and stability of explosive properties. The consensus standard also provides information on the procedures for classification of explosive materials.
https://www.unece.org/tans/danger/publi/manual/maual_e.html.
• Appendix B.2.3—Flammable Gases
○ ISO 10156:2010, Gases and Gas Mixtures—Determination of Fire Potential and Oxidizing Ability for the Selection of Cylinder Valve Outlets, Third Edition, April, 2010: This consensus standard provides specific details of the methods used to determine flammability of a gas or gas mixture. The standard also provides methods for determining if a gas or gas mixture is more or less oxidizing than air under atmospheric conditions. The intention of the standard is for classifying gases and gas mixtures as flammable gases and can be used to select the appropriate gas cylinder valve outlet for the flammability classification.
International Organization for Standards; https://iso.org/store.html.
○ ISO 817:2014 Refrigerants—Designation and safety classification: This consensus standard establishes a safety classification system based on the toxicity and flammability of the refrigerant. It also provides guidance on how to determine a refrigerant concentration limit.
International Organization for Standards; https://iso.org/store.html.
○ IEC 60079-20-1 ed. 1.0 (2010-01) Explosive atmospheres—Part 20-1: Material characteristics for gas and vapor classification—Test methods and data: This consensus standard provides guidance for classification of gas-air mixtures and vapor-air mixtures under normal conditions of pressure/temperature while also providing guidance on the appropriate selection of equipment. In addition, the standard provides guidance for determining the auto-ignition temperature of gas-air mixtures and vapor-air mixtures with additional information provided to guide selection of appropriate equipment for use in hazardous areas.
International Electrotechnical Commission: https://iec.ch/index/htm#buy.
○ DIN 51794 Determining the ignition temperature of petroleum products: This consensus standard provides detailed information on test methods used to determine the ignition temperature of petroleum products. The standard applies to flammable gases and liquids in a specific range of ignition temperature (75-650 °C) with particular emphasis on mineral oils hydrocarbons and their mixtures,
German Institute of Standards: https://din.de/en/about-standards/buy-standards.
○ UN ST/SG/AC.10/30/Rev.6, UN Recommendations on the Transport of Dangerous Goods, Manual of Tests and Criteria, Part III: This standard provides test methods for determining the flammability of aerosols and gases. The standard provides additional information on the criteria used in classifying gases with regards to their flammability.
https://www.unece.org/tans/danger/publi/manual/maual_e.html.
• Appendix B.4—Oxidizing Gases
○ ISO 10156: 2010, Gases and Gas Mixtures—Determination of Fire Potential and Oxidizing Ability for the Selection of Cylinder Valve Outlets, Third Edition, April, 2010: This consensus standard provides specific details of the methods used to determine flammability of a gas or gas mixture. The standard also provides methods for determining if a gas or gas mixture is more or less oxidizing than air under atmospheric conditions. The standard provides information on criteria that may be used for classifying gases and gas mixtures as flammable gases and may be used to select the appropriate gas cylinder valve outlet for the flammability classification.
https://www.iso.org/store.html.
• Appendix B.14.2—Oxidizing Solids
○ UN ST/SG/AC.10/30/Rev.6, UN Recommendations on the Transport of Dangerous Goods, Manual of Tests and Criteria, Part III: This section of the standard provides detailed test methods for determining the potential of a solid substance to increase the burning potential or burning intensity of a combustible substance when the two are thoroughly mixed. The standard also provides schematic with criteria on classifying solid substances based on the oxidizing potential.
https://www.unece.org/tans/danger/publi/manual/maual_e.html.
• Appendix B.17.2—Desensitized Explosives
○ UN ST/SG/AC.10/30/Rev.6, UN Recommendations on the Transport of Start Printed Page 9696 Dangerous Goods, Manual of Tests and Criteria, Part V: This section of the Manual identifies criteria for classification of desensitized explosives, and addresses the proper storage of these substances. The standard provides testing criteria and guidance on classifying, storing, and properly transporting goods according to their physical hazards.
https://www.unece.org/tans/danger/publi/manual/maual_e.html.
○ UN ST/SG/AC.10/30/Rev.6, UN Recommendations on the Transport of Dangerous Goods, Manual of Tests and Criteria, Part II: This standard provides information on the definition of desensitized explosives, the test methods used to evaluate a substance's ability to suppress its explosive properties when thoroughly mixed as a homogenous liquid and provides the criteria used to classify these substances based on their desensitizing properties.
https://www.unece.org/tans/danger/publi/manual/maual_e.html.
• Appendix B.17.3—Desensitized Explosives
○ UN ST/SG/AC.10/30/Rev.6, UN Recommendations on the Transport of Dangerous Goods, Manual of Tests and Criteria, Part II: This standard provides information on the definition of desensitized explosives, the test methods used to evaluate a substance's ability to suppress its explosive properties when thoroughly mixed as a homogenous liquid and provides the criteria used to classify these substances based on their desensitizing properties.
https://www.unece.org/tans/danger/publi/manual/maual_e.html.
The proposed inclusion of UN ST/SG/AC.10/30/Rev.6, UN Recommendations on the Transport of Dangerous Goods, Manual of Tests and Criteria, in some sections of appendix B (B.1, B.2, B.3, B.4, B.14, and B.17) would align with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060). However, an earlier version of UN ST/SG/AC.10 (Rev. 4) was incorporated by reference as part of the 2012 rulemaking and OSHA is not currently proposing to update all of the Rev. 4 references to Rev. 6 as part of this rulemaking. OSHA requests comments on whether it should, in the final rule, update all of the existing references to UN ST/SG/AC.10/30 to Rev.6 or add Rev. 6 references to the existing Rev. 4 references such that they would be alternative options for compliance.
UN ST/SG/AC.10/Rev.4 is included in the proposed regulatory text as part of the revision to the structure of § 1910.6(bb).
Copies of the standards are available for purchase from the issuing organizations at the addresses or through the other contact information listed in § 1910.6 for these private standards organizations. The UN documents are available at no cost through the contact information listed above. In addition, in accordance with § 1910.6(a)(4), these standards are available for inspection at any Regional Office of the Occupational Safety and Health Administration (OSHA), or at the OSHA Docket Office, U.S. Department of Labor, 200 Constitution Avenue NW, Room N-3508, Washington, DC 20210; telephone: 202-693-2350 (TTY number: 877-889-5627). Due to copyright issues, OSHA cannot post consensus standards on the OSHA website or through regulations.gov.
B. Regulatory Text
OSHA has proposed numerous revisions to the HCS regulatory text. The discussion of the proposed modifications is organized by paragraphs to the regulatory text with each modification/addition, and the reasons for and anticipated impact of each, described in detail below. Stakeholders can examine the redline strikeout version of the regulatory text at the OSHA HCS web page (https://www.osha.gov/dsg/hazcom/) or in the docket of this rulemaking (OSHA, 2020, Document ID 0222).
Paragraph (a) Purpose
Existing paragraph (a)(1) of the HCS states that the purpose of the standard is to ensure that the hazards of all chemicals produced or imported are classified, and that information concerning the classified hazards is transmitted to employers and employees. This provision currently explains that the requirements of the standard are intended to be consistent with the GHS Rev. 3. As the changes in this proposal would align the HCS with the GHS Revision 7, OSHA proposes to change the reference from Rev. 3 to Rev. 7.
Paragraph (b) Scope and Application
The scope section of the HCS identifies the chemicals that are (and are not) covered by the standard. Existing paragraph (b)(6)(x) excludes nuisance particulates from the standard where the chemical manufacturer or importer can establish that they do not pose any physical or health hazard covered by the standard. OSHA proposes a slight revision to this provision to make clear that nuisance particulates are excluded if they do not pose any physical hazard, health hazard, or other hazards (i.e., hazard not otherwise classified (HNOC)) covered by the standard. This proposal would clarify that all hazards covered by the standard must be considered when evaluating nuisance particulates.
Paragraph (c) Definitions
OSHA proposes to update three existing definitions and to add eight new terms and definitions to the HCS. In addition, the agency is proposing to eliminate one definition from the standard.
OSHA is proposing to add a definition of the term Bulk Shipment to the standard. The addition of this definition supports proposed paragraph (f)(5)(ii), which clarifies labeling requirements for bulk shipments of hazardous chemicals. The proposed definition would state that "bulk shipment" means any hazardous chemical transported where the mode of transportation (vehicle) comprises the immediate container (e.g., contained in tanker truck, rail car, or intermodal container).
OSHA is proposing to add the term Combustible Dust to the standard. In updating the HCS in 2012, OSHA did not include a definition of combustible dust because the agency was considering a combustible dust rulemaking and the UNSCEGHS was also considering combustible dust classification and communication issues (see 77 FR at 17705). However, OSHA has not promulgated a combustible dust standard. Since 2012, the UNSCEGHS has adopted a definition; the GHS Rev. 7 defines combustible dust as "finely divided solid particles of a substance or mixture that are liable to catch fire or explode on ignition when dispersed in air or other oxidizing media" (definition adopted from ISO/IEC 80079-20-2 as referenced in UN GHS, 2017, Document ID 0060). OSHA has preliminarily determined that this definition is consistent with existing OSHA guidance on combustible dust hazards and proposes to adopt this definition (OSHA, 2020, Document ID 0190; OSHA, 2009, Document ID 0255). OSHA has several standards that use the term "combustible dust," but do not define the term (e.g., § 1910.272, Grain Handling Facilities). OSHA believes the proposed definition of the term for the HCS is consistent with the use of that term in those other standards.
OSHA is also proposing to revise the definition of exposure or exposed. The definition currently provides, in relevant part, that exposure or exposed means that an employee is subjected in the course of employment to a chemical that is a physical or health hazard. OSHA proposes to revise the definition to mean an employee is subjected in the course of employment to a "hazardous chemical," rather than to "a chemical Start Printed Page 9697 that is a physical or health hazard," to clarify that the HCS covers the hazards of all hazardous chemicals, including those considered to be HNOCs.
OSHA is proposing to include three new definitions for the terms Gas, Liquid, and Solid. The agency is proposing to include these terms to align with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060). Although not included in the GHS, OSHA is proposing to add the temperature in equivalent degrees Fahrenheit and pressure in equivalent pounds per square inch (PSI) to the GHS definitions of gas and liquid because those measurements are more commonly used in the U.S.
Consistent with the GHS, OSHA proposes to define gas as a substance which (i) at 122 °F (50 °C) has a vapor pressure greater than 43.51 PSI (300 kPa) (absolute); or (ii) is completely gaseous at 68 °F (20 °C) at a standard pressure of 14.69 PSI (101.3 kPa). Also consistent with the GHS, OSHA proposes to adopt the definition of liquid as a substance or mixture which at 1220F (50 °C) has a vapor pressure of not more than 43.51 PSI (300 kPa (3 bar)), which is not completely gaseous at 680F (20 °C) and at a standard pressure of 101.3 kPa, and which has a melting point or initial melting point of 68 0F (20 °C) or less at a standard pressure of 14.69 PSI (101.3 kPa). Furthermore, in accordance with the GHS, OSHA is proposing to include the following as part of the definition of liquid: A viscous substance or mixture for which a specific melting point cannot be determined shall be subjected to ASTM D4359-90 (the Standard Test Method for Determining Whether a Material Is a Liquid or a Solid (2019)); or to the test for determining fluidity (penetrometer test) prescribed in section 2.3.4 of Annex A of the European Agreement concerning the International Carriage of Dangerous Goods by Road (ADR, 2019). Finally, OSHA proposes to adopt the GHS definition of solid as a substance or mixture which does not meet the definitions of liquid or gas.
Although OSHA did not include these terms in the 2012 update to the HCS, the agency is now proposing to include these definitions in order to improve clarity and ensure consistency in hazard communication both domestically and internationally. The agency anticipates that including these terms in the standard will clarify provisions under appendices B and D for classification of hazardous chemicals and preparation of SDSs. OSHA does not anticipate that these new definitions will impact other existing standards for construction or general industry. OSHA is requesting comments on its preliminary decision to include these definitions in this update.
OSHA is proposing to update the definition of hazardous chemical to delete the reference to pyrophoric gas because OSHA is proposing to classify this hazard as a physical hazard in the flammable gas hazard class (see discussion of proposed revisions to appendix B.2) and it is no longer necessary to list it separately in the definition. Concomitantly, OSHA is proposing to delete the separate definition for pyrophoric gas.
OSHA proposes to add a definition for immediate outer package to mean the first packaging enclosing the container of hazardous chemical. While all containers of chemicals must be labeled, as discussed in the Summary and Explanation for paragraph (f), below, OSHA is proposing revised labeling requirements for small containers. Proposed paragraph (f)(12) would relax labeling requirements for small containers, but would require complete label information on the "immediate outer package." For example, in the case of a kit, the container would be whatever surrounds the chemical itself (e.g., a vial), and the immediate outer package would be the first box or package surrounding the container.
The agency is also proposing to update the definition of physical hazard to mean a chemical that is classified as posing one of the following hazardous effects: Explosive; flammable (gases, liquids, or solids); aerosols; oxidizer (liquid, solid or gas); self-reactive; pyrophoric (liquid or solid); self-heating; organic peroxide; corrosive to metal; gas under pressure; in contact with water emits flammable gas; or desensitized explosive. The proposed definition also explicitly states that the criteria for determining whether a chemical is classified as a physical hazard are detailed in appendix B of the standard. The proposal would make two substantive changes to the current definition: (1) It would move the reference to aerosols out of the parenthetical following the word "flammable"; and (2) it would add a reference to desensitized explosives. These proposed revisions are intended to reflect the proposed new hazard classes for aerosols and desensitized explosives in appendix B in accordance with the GHS Rev. 7. These changes are discussed in greater detail in the Summary and Explanation of appendix B.
OSHA is proposing to add a definition of Physician or other licensed health care professional (PLHCP) to the standard. OSHA proposes to define this term as an individual whose legally permitted scope of practice (i.e., license, registration, or certification) allows the individual to independently provide or be delegated the responsibility to provide some or all of the health care services referenced in paragraph (i) of the standard. The new definition is necessary in light of OSHA's proposal to replace the phrase "physician and nurse" in paragraph (i), trade secrets with the term "PLHCP" to be consistent with other OSHA standards that use the term PLHCP, and to better reflect current medical practices. That change is discussed in greater detail in the Summary and Explanation of paragraph (i). OSHA believes the proposed definition of "PLHCP" is consistent with the way the agency has defined that term in all health standards promulgated since the bloodborne pathogen standard, 29 CFR 1910.1030, in 1991.
OSHA is also proposing to add a new definition, released-for-shipment, to mean a chemical that has been packaged and labeled in the manner in which it will be distributed or sold. This is a new term OSHA is proposing to use in paragraphs (f)(1) and (11) related to updating labels when new hazard information becomes available. OSHA notes that this definition is similar, but not identical to, the definition used by the U.S. Environmental Protection Agency's (EPA's) Pesticide Registration and Classification Procedures regulation, 40 CFR 152.3. EPA defines a product as released for shipment when the producer has packaged and labeled it in the manner in which it will be distributed or sold, or if it is stored in an area where finished products are ordinarily held for shipment. OSHA is not proposing to include chemicals that are stored in an area where finished products are usually held (but not packaged and labeled) in the definition of "released for shipment" because there do not appear to be any feasibility issues with ensuring that such chemicals are labeled with the most updated information. The agency is requesting comments on whether the proposed definition is appropriate for application to the HCS. OSHA is also interested in understanding whether the slight differences between OSHA's and EPA's definitions will pose any compliance issues for entities dealing with both OSHA and EPA labeling requirements. See the discussion of the proposed revisions to paragraph (f) for additional details.
Paragraph (d) Hazard Classification
OSHA is proposing two changes to paragraph (d)(1). OSHA proposes to revise the second sentence of paragraph Start Printed Page 9698 (d)(1) to read that for each chemical, the chemical manufacturer or importer shall determine the hazard classes, and where appropriate, the category of each class that apply to the chemical being classified under normal conditions of use and foreseeable emergencies. The language OSHA is proposing to add at the end of that sentence ("under normal conditions of use and foreseeable emergencies") simply reiterates the scope language currently in paragraph (b)(2) and OSHA's longstanding position that hazard classification must cover the normal conditions of use and foreseeable emergencies. As OSHA explained in its compliance directive for the HCS (OSHA, 2015, Document ID 0007), for example, known intermediates, by-products, and decomposition products that are produced during normal conditions of use or in foreseeable emergencies must be addressed in the hazard classification.
OSHA also proposes to add a new sentence to paragraph (d)(1) stating that the hazard classification shall include any hazards associated with a change in the chemical's physical form or resulting from a reaction with other chemicals under normal conditions of use. OSHA believes this language is necessary because there has been some confusion about whether chemical reactions that occur during normal conditions of use must be considered during classification. The agency's intent has always been to require information on SDSs that would identify all chemical hazards that workers could be exposed to under normal conditions of use and in foreseeable emergencies (see paragraph (b)(2)). This issue has been raised, for instance, when multiple chemicals are sold together with the intention that they be mixed together before use. For example, epoxy syringes contain two individual chemicals in separate sides of the syringe that are mixed under normal conditions of use. While OSHA intends for the hazards created by the mixing of these two chemicals to be considered in classification, those hazards need only appear on the SDS (see appendix D to § 1910.1200—Safety Data Sheets, section 3) and not on the label. For additional information, please see the Summary and Explanation for appendix D.
Paragraph (e) Written Hazard Communication Plan
OSHA is proposing a minor editorial correction in paragraph (e)(4). OSHA has found that an inadvertent misprint occurred in the print version of the CFR. Specifically, in the print version of the CFR, paragraph (e)(4) references § 1910.20 instead of § 1910.1020. Notably, this error is reflected only in the print version of the CFR; the eCFR (www.ecfr.gov) is correct. OSHA proposes to fix this error so that the print and electronic versions of the standard are the same.
Paragraph (f) Labels and Other Forms of Warning
Paragraph (f) of the HCS provides requirements for labeling. OSHA is proposing to modify paragraphs (f)(1), (5), and (11), and is also proposing a new paragraph (f)(12).
Paragraph (f)(1), Labels on shipped containers, currently specifies what information is required on shipped containers of hazardous chemicals and also provides that HNOCs do not have to be addressed on the containers. OSHA proposes to revise paragraph (f)(1) to provide that, in addition to HNOCs, hazards resulting from a reaction with other chemicals under normal conditions of use do not have to be addressed on shipped containers. OSHA believes this information is not appropriate on containers because it might confuse users about the immediate hazards associated with the chemical in the container. However, information on hazards resulting from a reaction with other chemicals under normal conditions of use is important for downstream users, and OSHA is not proposing to change the existing requirements for these hazards to be indicated on SDSs (under appendix D) and addressed in worker training where applicable (under paragraph (h)). OSHA also proposes to add the word "distributor" to the third sentence of paragraph (f)(1) to make it consistent with the first sentence.
In new paragraph (f)(1)(vii), OSHA is proposing to add a requirement that the label include the date a chemical is released for shipment. The agency is proposing this change in conjunction with changes in paragraph (f)(11) related to relabeling of containers that are released for shipment but have not yet been shipped. Providing the date a chemical is released for shipment on the label would allow manufacturers and distributors to more easily determine their obligations when new hazard information becomes available.
Paragraph (f)(5) specifies label requirements that apply to the transport of hazardous chemicals from workplace to workplace. OSHA proposes to add the heading "Transportation" to this paragraph and to add two new paragraphs to (f)(5) that specify requirements related to transportation of hazardous chemicals.
OSHA is proposing to add new paragraph (f)(5)(ii) to address the transport of bulk shipments of hazardous chemicals (e.g.,, in tanker trucks or rail cars). The proposed paragraph would specify that labels for bulk shipments of hazardous chemicals may either be on the immediate container or may be transmitted with shipping papers, bills of lading, or other technological or electronic means so that the information is immediately available in print to workers on the receiving end of the shipment. The proposed paragraph would codify policy from a 2016 guidance document that OSHA created jointly with DOT's Pipeline Hazardous Materials Safety Administration (PHMSA), with the intent of providing stakeholders with clarity for how to properly label bulk chemicals in transport (PHMSA, 2016, Document ID 0244). OSHA requests comments on whether it is appropriate to add proposed paragraph (f)(5)(ii) to the HCS and whether the addition of that paragraph would provide clarity regarding labeling of bulk chemical shipments.
Under the current HCS, appendix C, paragraph C.2.3.3 provides that where a pictogram required by the DOT appears on a shipped container, the HCS pictogram for the same hazard (specified in C.4) shall not appear. This provision was intended to prevent confusion associated with having two different representations of the same hazard on the container (77 FR 17728). However, after learning that DOT updated its regulations to indicate that it does not consider the HCS pictogram to conflict with the DOT pictogram, OSHA no longer believes that having both pictograms will create confusion for workers handling the chemical. Accordingly, OSHA proposes to: (1) Delete the language currently in paragraph C.2.3.3 from appendix C; and (2) adopt new paragraph (f)(5)(iii) to provide that where a DOT pictogram appears on a label for a shipped container, the appendix C pictogram for the same hazard is allowed, but is not required, on the HCS label.
For example, in the case where a chemical is shipped in only its immediate container, such as a 55-gallon drum containing a flammable liquid, both a DOT label and an OSHA-compliant label would be required. Under the current standard, the flame pictogram on the OSHA-compliant label would be prohibited because the DOT label would contain the equivalent pictogram. The proposed rule would allow, but not require, the flame pictogram to appear on the OSHA-compliant label. This means chemical Start Printed Page 9699 manufacturers could use the same labels for shipping containers and for containers that are solely used in the workplace; this would avoid information loss and eliminate the need to develop or print additional labels.
Paragraph (f)(11) currently requires that chemical manufacturers, importers, distributors, or employers who become newly aware of any significant information regarding the hazards of a chemical revise the labels within six months of becoming aware of the new information and ensure that labels on containers of hazardous chemicals shipped after that time contain the new information. OSHA recognizes that, on some occasions, a chemical manufacturer or importer may become aware of significant hazard information after a chemical has already been labeled but before it ships. Therefore, OSHA proposes to add a sentence to paragraph (f)(11) providing that chemicals that have been released for shipment and are awaiting future distribution need not be relabeled; however, the proposed sentence also states that the chemical manufacturer or importer must provide the updated label for each individual container with each shipment. The purpose of this proposal is to account for the long distribution cycles of some products and the potential hazards workers could face in relabeling the immediate containers of hazardous chemicals that have already been prepared for shipment.
Following publication of the 2012 updates to the HCS, OSHA received feedback related to difficulties some chemical manufacturers were having complying with paragraph (f)(11), particularly in the case of chemicals that travel through long distribution cycles (Kenyon, 2017, Document ID 0182). Many products have straightforward supply chains and are packaged, labeled, and promptly shipped downstream. Other products, for example in the agrochemical sector, are packaged and labeled when they leave the chemical manufacturer's facility, but they may reside at a warehouse or distribution facility for extended periods of time (e.g.,, several years) before being shipped downstream. There are also instances where products may be returned from the downstream users to the distribution facility and then shipped to other customers (NGFA, 2016, Document ID OSHA-2016-0005-0018; AFIA, 2016, Document ID OSHA-2016-0005-0017).
The act of relabeling these products in warehouses or distribution facilities has the potential to pose occupational safety and health risks to employees. Relabeling each individual container may require that employees open already secure packaging, a process that may result in potential chemical exposures. Furthermore, OSHA believes re-labeling of sealed hazardous chemical containers is not a common practice in warehouses and that warehouses may lack the equipment necessary to relabel products in a safe and effective manner.
OSHA has previously recognized the complexities involved with relabeling existing stock of hazardous chemicals. Following promulgation of the 2012 updates to the HCS, the HCS compliance directive (OSHA, 2015, Document ID 0007) provided enforcement guidance on the labeling of existing stock. Before June 1, 2015 (for manufacturers and importers), and before December 1, 2015 (for distributors), OSHA permitted chemical manufacturers, importers, and distributors with existing stock that was packaged (e.g.,, boxed, palletized, shrink wrapped, etc.) for shipment and labeled in accordance with the pre-2012 version of the HCS to ship those containers downstream without relabeling the containers with HCS 2012-compliant labels. However, the chemical manufacturer or importer generally had to provide an HCS 2012-compliant label for each individual container shipped and the appropriate HCS 2012-compliant SDS(s) with each shipment. After June 1, 2015, chemical manufacturers and importers of hazardous chemicals were required to ensure that each container was labeled with an HCS 2012-compliant label prior to shipping, and all distributors were generally required to ensure any chemicals shipped after December 1, 2015, were labeled in accordance with the 2012 updates to the HCS. OSHA used this enforcement policy as a basis for the proposed revisions to paragraph (f)(11).
OSHA has preliminarily determined that the proposed changes to paragraph (f)(11) would adequately address issues with long distribution cycles while still ensuring chemical users receive the most current hazard information. OSHA invites comments on the proposed revisions to this paragraph. In particular, OSHA requests comments on whether the proposed changes would adequately address issues associated with relabeling in cases of long distribution cycles, whether the proposed changes would provide sufficient flexibility, and whether the proposed revisions would alleviate safety concerns that would otherwise be associated with the relabeling of packaged stock.
OSHA is proposing a new paragraph, (f)(12), to address small container labeling. Currently, the HCS requires that all shipped containers be labeled with the information specified in paragraph (f)(1). The HCS, as updated in 2012, requires considerably more information on the label than the standard required previously; labels must include all hazards, as well as pictograms and precautionary statements. Many stakeholders have told OSHA that they have difficulties including all of the required information from paragraph (f)(1) on the labels they use for small containers. In some cases, the information becomes too small for a person to read it, and while it is sometimes possible to use alternate types of labels (such as pull-out labels or tags), it is not always feasible to do so (Watters, 2013, Document ID 0200; Collatz, 2015, Document ID 0174; Blankfield, 2017, Document ID 0170). In response to these concerns, through letters of interpretation and the HCS directive (OSHA, 2015, Document ID 0007; Watters, 2013, Document ID 0200; Collatz, 2015, Document ID 0174; Blankfield, 2017, Document ID 0170), OSHA provided a practical accommodation to address situations where it is infeasible to provide all HCS-required label information directly on small containers through the use of pull-out labels, fold-back labels, or tags. The practical accommodation allows limited information to be included on the small container label, but requires complete label information to be provided on the outside packaging. OSHA proposes to incorporate this practical accommodation into the standard in new paragraph (f)(12).
OSHA is proposing that all of the small container labeling provisions apply only where the chemical manufacturer, importer, or distributor can demonstrate that it is not feasible to use pull-out labels, fold-back labels, or tags containing the full label information required by paragraph (f)(1). Proposed paragraph (f)(12)(ii)(A) through (E) would provide that labels on small containers that are less than or equal to 100 milliliter (ml) capacity must include, at minimum: Product identifier; pictogram(s); signal word; chemical manufacturer's name and phone number; and a statement that the full label information for the hazardous chemical is provided on the immediate outer package. Additionally, proposed paragraph (f)(12)(iii) would provide that no labels are required for small containers of 3 ml capacity or less where the chemical manufacturer, importer, or distributor can demonstrate that any label would interfere with the normal use of the container; however, Start Printed Page 9700 that same proposed paragraph would state that if no label is required, the container must bear, at minimum, the product identifier. For example, the product identifier (e.g., CAS number) could be etched on a 3 ml glass vial (container) to ensure that the identifier remains fixed to the vial. This type of identification would ensure that the chemical in the small container can be identified and matched with the chemical's full label information.
Proposed paragraph (f)(12)(iv) would provide that for any small container covered by paragraph (f)(12)(ii) or (iii), the immediate outer package must include the full label information required by paragraph (f)(1) for each hazardous chemical in the immediate outer package, along with a statement that the small container(s) inside must be stored in the immediate outer package bearing the complete label when not in use. This proposed paragraph would also state that labels affixed to the immediate outer package must not be removed or defaced, as required by existing paragraph (f)(9).
OSHA believes that proposed paragraph (f)(12) would provide chemical manufacturers, importers and distributors with flexibility in labeling small containers. The proposed paragraph is consistent with the small packaging examples provided in the GHS Annex 7: Examples of Arrangements of the GHS Label Elements (UN GHS, 2016, Document ID 0197), and would result in better alignment with Health Canada's Hazardous Product Regulations (HPR) small capacity container requirements (Health Canada, 2015, Document ID 0051). Specifically, the HPR, under 5.4(1), provides exemptions from certain labeling requirements (such as precautionary statements) for small capacity containers of 100 ml or less. In addition, under 5.4(2), the HPR provides labeling exemptions for containers of 3 ml or less if the label interferes with the normal use of the hazardous product. OSHA requests comments on the feasibility of the proposed small container labeling provisions. The agency also requests feedback about whether the proposed changes would improve safe handling and storage for chemicals in small containers.
Paragraph (g) Safety Data Sheets
SDSs provide important safety information to employers and employees on the use of hazardous chemicals in the workplace. Additionally, SDSs provide detailed technical information and serve as a reference source for exposed employees, industrial hygienists, safety professionals, emergency responders, health care professionals, and other interested parties. While OSHA believes that information in SDSs has greatly improved with the standardized, 16-section format prescribed in the 2012 updates to the HCS, the agency is proposing two minor changes to paragraph (g) to ensure consistency and accessibility of the SDSs.
The proposed revisions to paragraph (g) are confined to paragraphs (g)(2) and (10). The purpose of paragraph (g)(2) is to identify what information must be included on an SDS. The first part of existing paragraph (g)(2) states that the chemical manufacturer or importer preparing the SDS shall ensure that it is in English. However, as permitted by paragraph (g)(1), some chemical manufacturers and importers may obtain, rather than prepare, SDSs. To minimize any potential confusion between paragraphs (g)(1) and (2), OSHA is proposing to revise paragraph (g)(2) by removing the reference to preparing the SDS. The first part of the first sentence in paragraph (g)(2) would be revised to read simply that the chemical manufacturer or importer shall ensure that the SDS is in English. This is a technical clarification intended to ensure consistency with paragraph (g)(1).
Paragraph (g)(10) addresses the form and storage of SDSs. The original intent of paragraph (g)(10) was to allow employers alternatives to SDSs within a plant site (see 48 FR 53337). Alternatives to SDSs, such as written operating procedures and manuals, are generally permitted. Existing paragraph (g)(10) also permits employers to design SDSs to cover groups of hazardous chemicals in a work area where it may be more appropriate to address the hazards of a process rather than individual chemicals. In any case, paragraph (g)(10) requires the employer to ensure that the required information is provided for each hazardous chemical and is readily accessible to employees. However, with the update to the HCS in 2012, OSHA changed the requirements of the SDS from a performance-oriented format to a standardized format. Standardizing the SDS format improved hazard communication by ensuring users could quickly find relevant information (see 77 FR 17596-98). Because SDSs now have a standardized format and are specific to individual hazardous chemicals, they are not permitted to be designed to cover groups of hazards, as currently provided in paragraph (g)(10). Therefore, OSHA is proposing a change to paragraph (g)(10) that would allow SDSs to be stored, rather than designed, in a way to cover groups of hazardous chemicals in a work area. OSHA believes that this change would allow employers flexibility in how they keep SDSs in the workplace while also ensuring that the mandatory 16-section SDS is maintained. The agency is requesting comments regarding whether this proposed revision would require stakeholders to make any significant changes to their current practices.
Paragraph (i) Trade Secrets
This paragraph describes certain conditions under which a chemical manufacturer, importer, or employer may withhold the specific chemical identity (e.g., chemical name), other specific identification of a hazardous chemical, or the exact percentage (concentration) of the substance in a mixture, from the SDS. OSHA is proposing three significant changes within paragraph (i)(1) and the paragraphs thereunder. First, OSHA is proposing to revise paragraph (i)(1) to allow for concentration ranges to be claimed as a trade secret and to specify that it is section 3 of the SDS from which trade secret information may be withheld.
Second, OSHA is proposing new paragraph (i)(1)(iv), which would require that when an ingredient's exact concentration or concentration range is claimed as a trade secret, the SDS must provide the ingredient's concentration as a concentration range selected from a prescribed list of ranges. These ranges are in proposed paragraphs (i)(1)(iv)(A) through (M) as follows: (1) From 0.1% to 1%; (2) from 0.5% to 1.5%; (3) from 1% to 5%; (4) from 3% to 7%; (5) from 5% to 10%; (6) from 7% to 13%; (7) from 10% to 30%; (8) from 15% to 40%; (9) from 30% to 60%; (10) from 45% to 70%; (11) from 60% to 80%; (12) from 65% to 85%; and (13) from 80% to 100%. These ranges are consistent with those used in Canada, first described under the WHMIS 1988 Controlled Products Regulation (CPR) and re-implemented in 2018 under the HPR (Canadian Gazette II, 2018, Document ID 0101). Using the same concentration ranges as Canada, one of the U.S.'s major trading partners, is part of the two countries' efforts through the Regulatory Cooperation Council to align hazard communication to the greatest extent possible.
OSHA has received numerous inquiries about the use of trade secrets for concentration ranges (Colau, 2017, Document ID 0098; Nelson, 2017, Document ID 0099). Although chemical manufacturers and importers are permitted to use concentration ranges rather than an exact percentage on the Start Printed Page 9701 SDS when there is batch-to-batch variability in the production of a mixture or for a group of substantially similar mixtures with similar chemical composition, OSHA does not currently allow trade secret status for a concentration range (see 77 FR 17731). However, in response to feedback from stakeholders who have indicated that there are instances where a concentration range is also a trade secret, OSHA has preliminarily determined it is appropriate to permit concentration ranges to be claimed as trade secrets as long as the ranges prescribed in proposed paragraphs (i)(1)(iv)(A) through (M) are used (Nelson, 2017, Document ID 0099; Colau, 2017, Document ID 0098).
Third, proposed new paragraph (i)(1)(v) would require that the concentration range used on the SDS be the narrowest range possible. This proposed paragraph would also provide that if the actual concentration range falls between 0.1% and 30% and does not fit entirely into one of the prescribed ranges in proposed paragraphs (i)(1)(iv)(A) through (G), a single range created by the combination of two applicable consecutive ranges between (i)(1)(v)(A) and (G) may be disclosed instead, provided that the combined concentration range does not include any range that falls entirely outside the exact range in which the ingredient is present. For example, a chemical manufacturer that wishes to claim the concentration of a specific ingredient (e.g., 2.5%) as a trade secret would have to use the prescribed range in proposed paragraph (i)(1)(iv)(C) of 1% to 5%. If the ingredient is in the mixture at a concentration range of 0.9% to 2%, then the chemical manufacturer could combine the prescribed ranges in proposed paragraphs (i)(1)(iv)(B) and (C), resulting in a range of 0.5% to 5% on the SDS. If the ingredient is in the mixture at a concentration range of 5% to 7%, the chemical manufacturer would have to use the range in proposed paragraph (i)(1)(iv)(D) of 3% to 7%, because it is narrower than the range in proposed paragraph (i)(1)(iv)(E) of 5% to 10%.
OSHA is requesting comments on the proposed revisions to paragraph (i)(1). Specifically, the agency is interested in any experience stakeholders have had with developing SDSs using the prescribed concentration ranges and any concerns stakeholders have about using concentration ranges on SDSs. The agency is also requesting comments addressing the adequacy of hazard information provided by these ranges. Do these ranges provide sufficient information for downstream chemical manufacturers to conduct hazard classifications? Are the ranges listed in proposed paragraphs (i)(1)(iv)(A) through (M) too wide (should they be narrowed)? Should OSHA allow combinations among all ranges (e.g., (i)(1)(v)(A) through (M)) or should the allowance for combining ranges be even more restrictive than proposed (e.g., (i)(1)(v)(A) through (E))?
OSHA is also proposing other changes in paragraph (i) to reflect the proposal to permit concentration ranges to be claimed as trade secrets and to adopt the "PLHCP" terminology in lieu of references to "physician or nurse." See discussion of proposed changes to paragraph (c), Definitions, where OSHA explains that it is proposing to replace the phrase "physician and nurse" with "PLHCP" to be consistent with other OSHA standards and to better reflect current medical practices. The specific changes OSHA is proposing are as follows:
- OSHA is proposing to revise paragraph (i)(1)(iii) to change "percentage" to "concentration or concentration range."
- OSHA is proposing to move existing paragraph (i)(1)(iv) to paragraph (i)(1)(vi) and to change "percentage" to "exact concentration or concentration range."
- In paragraph (i)(2), OSHA is proposing to change "physician or nurse" to "PLHCP" and to replace "percentage of composition" with "concentration or concentration range."
- OSHA is proposing to revise paragraph (i)(3) to change "percentage composition" to "exact concentration or concentration range" and to change the parenthetical from "(i.e., physician, industrial hygienist, toxicologist, epidemiologist, or occupational health nurse)" to "(e.g., PLHCP, industrial hygienist, toxicologist, or epidemiologist)."
Paragraph (j) Dates
OSHA is proposing to implement the revised provisions over a two-year phase-in period. OSHA proposes that the revisions become effective 60 days after the publication date (paragraph (j)(1)) and that chemical manufacturers, importers, and distributors evaluating substances comply with all modified provisions of the HCS no later than one year after the effective date (paragraph (j)(2)). OSHA also proposes that chemical manufacturers, importers, and distributors evaluating mixtures comply with all modified provisions no later than two years after the effective date (paragraph (j)(3)).
Proposed paragraph (j) would replace the regulatory text currently in paragraph (j), as the dates specified in existing paragraph (j) have all passed. This proposed paragraph is based in part on stakeholder comments and the agency's experience implementing the 2012 updates to the HCS. In 2012, OSHA did not stagger the compliance dates for substances and mixtures; however, OSHA believes that such a tiered approach may ease the compliance burden for manufacturers of mixtures that may rely on the hazard information in the SDSs from their ingredient suppliers to update the labels and SDSs for the mixtures. The changes OSHA is proposing in this update are far less complicated than the 2012 revision and would result in no change in hazard classification for the vast majority of chemicals. Additionally, the proposed update to paragraph (f)(11) addressing relabeling requirements for chemicals that have been released for shipment would also reduce the need for a lengthier implementation period. OSHA is requesting comments regarding the adequacy and appropriateness of the proposed compliance dates and on the feasibility of implementing a tiered compliance approach for substances and mixtures.
C. Appendix A
OSHA is proposing to update appendix A in several respects. The proposed changes are discussed in order of revisions to specific health hazards in appendix A, followed by general changes to definitions and terminology, clarification of mandatory requirements, and corrections. OSHA preliminarily concludes that all of the proposed changes to appendix A will improve classification and communication of hazards and thus better protect workers. Many of the proposed changes would align the HCS with the GHS Rev. 7. Aligning the HCS with the GHS would ease compliance burdens for U.S. stakeholders who must also comply with international requirements for hazard classification and communication.
OSHA is providing a redline strikeout version of appendix A, which reflects all of OSHA's proposed revisions, in the docket and on the OSHA website (OSHA HCS Redline, 2020, Document ID 0222; https://www.osha.gov/dsg/hazcom/). This will allow interested parties to view all of the proposed changes in context. OSHA strongly encourages stakeholders to review that document in conjunction with the discussion of the proposed revisions below, as the discussion below does not fully describe all of the non-substantive or editorial changes OSHA is proposing. Start Printed Page 9702
Revisions to Health Hazards in Appendix A
General Classification Considerations
In Paragraph A.0.1, OSHA proposes to add a note from Paragraph 1.3.3.1.3 of the GHS Rev. 7 (UN GHS, 2017, Document ID 0060), providing that where impurities, additives, or individual constituents of a substance or mixture have been identified and are themselves classified, they should be taken into account during classification if they exceed the cut-off value/concentration limit for a given hazard class. OSHA did not include this note in the HCS in 2012 because the definition of "substance" in paragraph (c) references additives and impurities, and therefore the classification of substances necessarily takes impurities and additives into account. Nonetheless, the agency now believes that this note is useful to align with the GHS and has added this note as proposed A.0.1.3. Including this provision would clarify that manufacturers and importers must consider the hazards of all classified components when classifying chemicals. This would help ensure accurate classification of chemicals and therefore improve protections for workers.
OSHA also proposes to modify the introduction of paragraph A.0.4.1 to include mandatory language. The current text indicates that the sequence in the process of classification of mixtures is recommended. OSHA proposes to revise A.0.4.1 to read "Except as provided in A.0.4.2, the process of classification of mixtures is based on the following sequence" to specify that this process is mandatory.
Acute Toxicity—(Appendix A.1)
In appendix A.1, OSHA proposes to revise the definition of acute toxicity to refer to serious adverse health effects (i.e., lethality) occurring after a single or short-term oral, dermal, or inhalation exposure to a substance or mixture. (The current definition refers to adverse effects occurring following oral or dermal administration of a single dose of a substance, or multiple doses given within 24 hours, or an inhalation exposure of 4 hours.) This change is being proposed to align with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2016, Document ID 0131).
OSHA also proposes to revise the classification criteria for substances in A.1.2.1 to note that while some in vivo methods determine LD50/LC50 values directly, other newer in vivo methods (e.g., using fewer animals) consider other indicators of acute toxicity, such as significant clinical signs of toxicity, which are used by reference to assign the hazard category. This change is being proposed to align with classification criteria in the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2016, Document ID 0131).
OSHA is also proposing slight revisions to Table A.1.1, "Acute toxicity hazard categories and acute toxicity estimate (ATE) values defining the respective categories", to align with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2016, Document ID 0131). The GHS presents the ATE range in Table A.1.1 using the term "ATE" to express the range, while the HCS currently uses the term "AND." Therefore, OSHA proposes to change the "AND" in the acute toxicity estimate (ATE) ranges to "ATE" to align with the GHS Rev. 7. This modification would not change the classification criteria itself, but would be more technically accurate and consistent with the way the table is expressed in the European Commission's (EC) Classification, Labelling, and Packaging of Substances and Mixtures (CLP) regulation (ECHA, 2017, Document ID 0256).
In paragraph A.1.2.3, OSHA proposes to include a new sentence at the end of the paragraph to clarify that both data from animal tests and human studies should be considered in evaluating acute toxicity. The new text states that in cases where data from human experience (i.e., occupational data, data from accident databases, epidemiology studies, clinical reports) is also available, it should be considered in a weight of evidence approach consistent with the principles described in A.0.3. To ensure human data is considered in classifying chemicals for all acute toxicity hazard categories, the GHS added this clarifying text in paragraph 3.1.2.3 (UN GHS, 2016, Document ID 0131). OSHA is proposing these changes to paragraph A.1.2.3 to align with the GHS Rev. 7.
OSHA also proposes a new paragraph A.1.2.4, which is intended to correspond to Chapter 3.1, (paragraph 3.1.2.6.5) in the GHS Rev. 7 (UN GHS, 2017, Document ID 0060). This proposed paragraph would provide that in addition to classification for inhalation toxicity, if data are available that indicate that the mechanism of toxicity was corrosivity of the substance or mixture, the classifier must consider whether the chemical is corrosive to the respiratory tract. This proposed paragraph would clarify that the hazard corrosive to the respiratory tract is covered under the HCS. OSHA did not explicitly include the corrosive to the respiratory tract hazard in the HCS in 2012, but explained in OSHA 3844: Hazard Communication: Hazard Classification Guidance for Manufacturers, Importers and Employers (OSHA, 2016, Document 0008) that this hazard should be considered during classification. The Hazard Classification guidance explains that if the classifier has data indicating that there is acute inhalation toxicity with corrosion of the respiratory tract that leads to lethality, then the substance or mixture may be labeled with the additional hazard statement "corrosive to the respiratory tract." However, if the classifier has data that indicates acute inhalation toxicity with corrosion of the respiratory tract and the effect does not lead to lethality, then the hazard may be addressed in the Specific Target Organ Toxicity hazard classes (see appendices A.8 and A.9). OSHA is including these clarifications in proposed A.1.2.4.1 and A.1.2.4.2, but is modifying the "may" language from the guidance to "must" language to ensure that corrosive to the respiratory tract is appropriately considered during the classification process.
In Figure A.1.1 and paragraph A.1.3.6.2.2, OSHA proposes to correct the cross-reference from A.1.3.6.2.3 to A.1.3.6.2.4. OSHA also proposes to amend paragraph A.1.3.6.2.3. If a mixture contains an ingredient of unknown acute toxicity at a concentration of at least 1 percent, paragraph A.1.3.6.2.3 currently requires a statement that "X" percent of a mixture consists of ingredient(s) of unknown toxicity on the label and SDS. OSHA proposes to revise this paragraph to require the statement to differentiate by route of exposure. For example, the statement(s) could read, "x % of the mixture consists of ingredient(s) of unknown acute oral toxicity" or "x % of the mixture consists of ingredient(s) of unknown acute dermal toxicity." Given that it is possible to have unknown ingredients for more than one relevant route of exposure (e.g., oral, dermal, inhalation), differentiating the statement by route would be helpful to chemical users. This proposed change would align with paragraph 3.1.3.6.2.2 in the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2010, Document 0089).
OSHA also proposes to delete the second paragraph in A.1.3.6.2.3 because it is duplicative of the first paragraph.
Skin Corrosion/Irritation and Serious Eye Damage/Eye Irritation—(Appendices A.2 and A.3)
OSHA is proposing more extensive revisions to the sections on skin corrosion/irritation and serious eye Start Printed Page 9703 damage/irritation (appendices A.2 and A.3) than it is proposing for the other health hazard sections in appendix A of the HCS. These two sections correspond to Chapters 3.2 and 3.3 in the GHS. The UNSCEGHS, in its 16th Session, assembled an informal working group to review the content of Chapters 3.2 and 3.3 in the GHS, and to propose editorial revisions in order to enhance clarity and user-friendliness in the application of the criteria (UN GHS, 2016, Document 0131). The group's primary focus was to change the order of the text to ensure that the classification strategy was clear, and to change the testing scheme to more of an evaluation scheme, since the GHS, like the HCS, is test method neutral. The work of the informal working group was not complete before OSHA published its updates to the HCS in 2012. The working group has since completed its efforts to clarify the skin corrosion/irritation and serious eye damage/irritation chapters. The work was approved by the UNSCEGHS in 2012 (UN GHS, 2012, Document ID 0212). Accordingly, OSHA is now proposing to revise appendices A.2 and A.3 to incorporate all of the modifications that were made to the GHS skin corrosion/irritation and serious eye damage/irritation chapters agreed to by the UNSCEGHS up to and including the GHS Rev. 7. This would ensure that OSHA remains aligned with the GHS. OSHA is not proposing any completely new provisions for the HCS; however, OSHA is proposing to revise the two appendices to align the language and format of the HCS with the GHS Rev. 7.
In appendix A.2, skin corrosion/irritation, OSHA proposes to modify paragraph A.2.1.2 to clarify the sequence in which data should be evaluated when classifying for skin corrosion/irritation using a tiered evaluation approach. The proposal would align the language in this paragraph with the tiered approach in Figure A.2.1. The first tier is existing human data, followed by existing animal data, followed by in vitro data, and then other sources of information.
The proposed changes to the skin corrosion/irritation criteria in paragraph A.2.2 are mainly editorial in nature. The classification criteria would remain the same, but the presentation of the information would be rearranged in a clearer, more logical fashion. In addition, OSHA is proposing new paragraph A.2.2.2.2, which is intended to provide classifiers with factors to be taken into consideration when evaluating irritant responses.
The proposed changes in paragraph A.2.3 are also mainly editorial in nature. The criteria would remain the same, but clarifying text would be introduced into the section and the criteria would be presented in a more logical sequence.
OSHA also proposes to include a new note to Table A.2.3, "Concentration of ingredients of a mixture classified as skin Category 1 or 2 that would trigger classification of the mixture as hazardous to skin (Category 1 or 2)," to indicate how to classify the mixture when data are available for sub-categorization of Category 1. The proposed note would align with the note to Table 3.2.3 in the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2012, Document ID 0132), and OSHA believes that it provides useful information for classifiers.
Figure A.2.1, "Tiered evaluation of skin corrosion and irritation potential", would remain largely the same under OSHA's proposed revisions to the appendix. However, OSHA is proposing to revise the title to "Tiered evaluation for skin corrosion and irritation." OSHA is also proposing to revise Steps 1a, 1b, and 1c of Figure A.2.1 to clarify that the parameter being evaluated is existing human or animal skin corrosion/irritation data. In addition, OSHA is proposing to modify the finding in Step 4 to clarify that high acid/alkaline reserve or no data for acid/alkaline reserve should be considered when the pH is ≤2 or ≥11.5. OSHA is also proposing some revisions to the footnotes of Figure A.2.1.
- In proposed footnote (1), OSHA is proposing to revise the current footnote to include an additional sentence indicating that although human data from accident or poison center databases can provide evidence for classification, absence of incidents is not itself evidence for a not classified determination. In addition, the reference to evidence from ethically-conducted human clinical studies would be removed. The text indicating that there is no internationally accepted test method for human skin irritation testing would also be removed.
- In proposed footnote (3), OSHA is proposing to revise the existing note to exclude the examples currently provided.
- In proposed footnote (6), OSHA is proposing to revise the current note to clarify that all available information on a substance must (instead of should) be considered in making a determination based on the total weight of evidence. OSHA is also proposing a new sentence at the end of the footnote to indicate that negative results from applicable validated skin corrosion/irritation in vitro tests are considered in the total weight of evidence evaluation.
In paragraph A.2.4, OSHA is proposing to include in A.2.4.1.1 language stating that the tiered approach must be taken into account when evaluating mixtures. In addition, a new paragraph A.2.4.1.2 is proposed to indicate that when considering testing of mixtures, classifiers must use the tiered approach to help ensure an accurate classification, as well as to avoid unnecessary animal testing. This proposed paragraph also indicates that if there are no other data on the mixture besides pH, and the pH is extreme (pH ≤2 or pH ≥11.5), that information is sufficient to classify the mixture as corrosive to the skin. However, if the acid/alkaline reserve suggests that the mixture may not be corrosive despite the extreme pH, then further evaluation may be necessary.
In Table A.2.4, "Concentration of ingredients of a mixture for which the additivity approach does not apply, that would trigger classification of the mixture as hazardous to skin," OSHA proposes to delete the phrase "for which additivity does not apply" where it appears in the text of the table in order to reduce redundancy, as that language is already included in the title of the table. However, OSHA is proposing to modify the title of Table A.2.4 from "for which additivity does not apply" to "when additivity does not apply" to be consistent with the GHS Rev.7 (UN GHS, 2017, Document ID 0060).
In appendix A.3, serious eye damage/eye irritation, OSHA proposes to modify A.3.1.2 to clarify the sequence in which data should be evaluated when classifying for serious eye damage/eye irritation using a tiered evaluation approach. The proposal would align the language in this paragraph with the tiered approach in Figure A.3.1. The first tier is existing human data, followed by existing animal data, followed by in vitro data, and then other sources of information.
The changes OSHA is proposing in paragraphs A.3.2 and A.3.3, including Tables A.3.1 and A.3.2, are mainly editorial in nature. The classification criteria in these paragraphs would remain the same, but the presentation of the information would be rearranged and additional headings would be included to provide a clearer, more logical sequence. All of these proposed changes would conform with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2012, Document 0132).
Proposed paragraph A.3.2 provides a summary of the classification criteria for substances that is provided in Tables A.3.1 and A.3.2. In addition, proposed paragraph A.3.3.6 is a reorganization of existing paragraphs A.3.3.3 and A.3.3.4. Start Printed Page 9704 It would provide guidance on using the tiered approach and making weight of evidence decisions and also indicates a preference for not conducting new animal tests.
Under OSHA's proposed revisions, Figure A.3.1, "Tiered Evaluation for serious eye damage and eye irritation", currently titled "Evaluation strategy for serious eye damage and eye irritation", would remain largely the same. However, as in Figure A.2.1, OSHA is proposing to revise Steps 1a, 1b, and 1c to clarify that the parameter being evaluated is existing human or animal serious eye damage/eye irritation data. In addition, OSHA is proposing to modify the finding in Step 4 to clarify that high acid/alkaline reserve or no data for acid/alkaline reserve should be considered when the pH is ≤2 or ≥11.5. OSHA is also proposing modifications to the footnotes of Figure A.3.1 to reflect the most recent test methods.
- In proposed footnote (3), OSHA is proposing to include an additional sentence that emphasizes that expert judgement should be exercised when making determinations from existing animal data indicating serious eye damage/eye irritation, as not all skin irritants are eye irritants.
- In proposed footnote (4), OSHA is proposing to include OECD Test Guideline 460 (Fluorescein leakage (FL) as an additional example of an internationally accepted, scientifically validated test method for identifying eye corrosives and severe irritants. OSHA is also proposing an additional sentence for this footnote to indicate that there are presently no scientifically validated and internationally accepted in vitro test methods for identifying eye irritation.
- In proposed footnote (6), OSHA is proposing to revise existing language to make it clear that all available information on a substance must (instead of should) be considered in making a determination based on the total weight of evidence. In addition, OSHA is proposing to add two new sentences at the end of the footnote to indicate that the total weight of evidence, including information on skin irritation, may lead to classification for eye irritation and that negative results from applicable scientifically validated in vitro tests are considered in the total weight of evidence evaluation.
In paragraph A.3.4, OSHA is proposing several minor editorial changes to ensure consistency in the terminology used. For example, OSHA is proposing to use the term "serious eye damage" (rather than "eye corrosion") throughout the text to reflect the name of the hazard class.
Germ Cell Mutagenicity—(Appendix A.5)
OSHA is proposing to add a definition for germ cell mutagenicity in A.5.1.1 explaining that germ cell mutagenicity refers to heritable gene mutations, including heritable structural and numerical chromosome aberrations in germ cells occurring after exposure to a substance or mixture. OSHA is proposing this definition to align with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2016, Document ID 0131). Because of this new paragraph, the subsequent numbering of existing paragraphs in A.5.1 would be adjusted accordingly.
In A.5.4, Examples of scientifically validated test methods, paragraph A.5.4.2, OSHA proposes to delete the Mouse spot test (OECD 484) as an example of an in vivo somatic cell mutagenicity test, as it was deleted by the OECD on April 2, 2014. This change is consistent with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2014, Document 0087) and ensures that hazard classifications are being conducted with the most current scientific principles.
Reproductive Toxicity—(Appendix A.7)
In appendix A.7, Reproductive toxicity, OSHA proposes to revise the "effects on or via lactation" hazard category classification criteria to align with OSHA publication 3844 Hazard Classification Guidance for Manufacturers, Importers and Employers (OSHA 3844, 2016, Document 0008). During the development of the guidance document, it became apparent to OSHA that there were issues with regard to the classification criteria in existing Figure A.7.1(b). The hazard category for effects on or via lactation captures two separate effects:
i. Substances that can interfere with lactation; and
ii. substances and their metabolites that may be transmitted through breast milk to children in amounts sufficient to cause concern for the health of the breast feeding child.
However, the current criteria do not adequately distinguish between these two separate effects. The first issue has both grammatical and substantive aspects and is found in the second sentence of Figure A.7.1(b), which currently reads:
"Chemicals that are absorbed by women and have been shown to interfere with lactation or that may be present (including metabolites) in breast milk in amounts sufficient to cause concern for the health of a breastfed child, shall be classified to indicate this property hazardous to breastfed babies."
The italicized phrase is not grammatically correct and is also not correct as a matter of substance because it ignores the effects on lactation. As such, OSHA proposes to delete the text to indicate this property "hazardous to breastfed babies." In addition, the categories of evidence currently listed in paragraphs (a) through (c) of Figure A.7.1(b) all provide evidence for effects via lactation rather than effects on lactation. To be more accurate, and to avoid confusion on how to apply the criteria for effects on lactation, OSHA proposes to modify the third sentence in the Figure to read: "Classification for effects via lactation shall be assigned on the basis of:" These proposed changes would not affect the classification of substances or mixtures as reproductive toxicants; however, they would be more accurate and provide more clarity for classifiers.
OSHA proposes to modify paragraph A.7.2.5.1 to include OECD Test Guideline 443, Extended One Generation Reproductive Toxicity Study, as an additional method for one or two generation toxicity testing. Additionally, in Table A.7.1 "Cut-off values/concentration limits of ingredients of a mixture classified as reproductive toxicants or for effects on or via lactation that trigger classification of the mixture", OSHA is proposing a correction to the top left heading from "ingredients classified as" to "ingredient classified as." OSHA believes that the use of the word "ingredients" in this context may be confusing, as it may suggest that the additivity principle should be applied. Therefore, OSHA is proposing this change for clarity. These proposed modifications in appendix A.7 are consistent with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2014, Document ID 0221).
Specific Target Organ Toxicity-Single Exposure—(Appendix A.8)
In appendix A.8, OSHA proposes to make a correction to paragraph A.8.1.6 to correctly name the hazard class as "specific target organ toxicity—single exposure" instead of "specific organ systemic toxicity single exposure." Also, in paragraph A.8.2.1.7.3, OSHA proposes to delete the erroneous inclusion of the second use of the word "evidence" in the second sentence.
OSHA proposes to include the concept of "relevant ingredient" when classifying mixtures containing Category 3 ingredients using the additivity approach. Under the HCS, as updated in Start Printed Page 9705 2012, the additivity principle was introduced in paragraph A.8.3.4.5. However, a "relevant ingredient" for this procedure had not been established. Proposed new paragraph A.8.3.4.6 would provide that in cases where the additivity approach is used for Category 3 ingredients, the "relevant ingredients" of a mixture are those which are present in concentrations ≥1% (w/w for solids, liquids, dusts, mists, and vapors and v/v for gases), unless there is a reason to suspect that an ingredient present at a concentration <1% is still relevant when classifying the mixture for respiratory tract irritation or narcotic effects. This proposed paragraph would align with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2014, Document 0221).
Aspiration Hazard—(Appendix A.10)
The proposed changes to appendix A.10 would provide clarification on the classification criteria for mixtures when data are available for all ingredients or only for some ingredients. OSHA is proposing new paragraph A.10.3.3.1 to clarify that the concept of "relevant ingredient" applies and that relevant ingredients are those that are present in concentrations of at least 1%. In addition, a new heading, "Category 1," is proposed as new paragraph A.10.3.3.2. Proposed A.10.3.3.2.1 and A.10.3.3.2.2 would clarify that the principle of additivity applies in appendix A.10, but OSHA is not proposing any substantive changes to the classification criteria. These proposed changes would align with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2014, Document 0221).
Changes to Definitions and Terminology, Clarification of Mandatory Requirements, and Corrections
Definitions
OSHA proposes to update appendix A to include changes to the health hazard definitions to reflect those adopted by the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2016, Document ID 0131). Since OSHA revised the HCS in 2012, the UNSCEGHS has revised all of the health hazard definitions in the GHS. These modifications to the health hazard definitions were incorporated as a result of the work of the UNSCEGHS Practical Classification Issues working group. The scope of the working group is to clarify the GHS classification criteria, as appropriate, and to provide working examples to illustrate application of the criteria. The previous health hazard definitions were not consistent with respect to form or content, and many of the definitions were taken directly from the OECD test guidelines.
The UNSCEGHS determined that the definitions should be more general and neutral with respect to test guidelines and that test guideline criteria should not be part of a definition. The group also determined that the health hazard definitions should be clear and concise and that there should be a clear differentiation between "definitions" and "general considerations" text. OSHA is proposing to adopt all of the revised health hazard definitions from the GHS Rev. 7 in appendix A, as well as corresponding changes to text throughout the appendix. For example, in some cases OSHA is proposing to remove OECD test guidelines from definitions and to move them to paragraphs outlining classification criteria. The health hazard definitions that OSHA is proposing in appendix A are:
- Acute toxicity refers to serious adverse health effects (i.e., lethality) occurring after a single or short-term oral, dermal, or inhalation exposure to a substance or mixture.
- Skin corrosion refers to the production of irreversible damage to the skin; namely, visible necrosis through the epidermis and into the dermis occurring after exposure to a substance or mixture.
- Skin irritation refers to the production of reversible damage to the skin occurring after exposure to a substance or mixture.
- Serious eye damage refers to the production of tissue damage in the eye, or serious physical decay of vision, which is not fully reversible, occurring after exposure of the eye to a substance or mixture.
- Eye irritation refers to the production of changes in the eye, which are fully reversible, occurring after exposure of the eye to a substance or mixture.
- Respiratory sensitization refers to hypersensitivity of the airways occurring after inhalation of a substance or mixture.
- Skin sensitization refers to an allergic response occurring after skin contact with a substance or mixture.
- Germ cell mutagenicity refers to heritable gene mutations, including heritable structural and numerical chromosome aberrations in germ cells occurring after exposure to a substance or mixture.
- Carcinogenicity refers to the induction of cancer or an increase in the incidence of cancer occurring after exposure to a substance or mixture.
- Reproductive toxicity refers to adverse effects on sexual function and fertility in adult males and females, as well as developmental toxicity in the offspring, occurring after exposure to a substance or mixture.
- Specific target organ toxicity-single exposure (STOT-SE) refers to specific, non-lethal toxic effects on target organs occurring after a single exposure to a substance or mixture.
- Specific target organ toxicity-repeated exposure (STOT-RE) refers to specific toxic effects on target organs occurring after repeated exposure to a substance or mixture.
- Aspiration hazard refers to severe acute effects such as chemical pneumonia, pulmonary injury or death occurring after aspiration of a substance or mixture.
- Aspiration means the entry of a liquid or solid chemical directly through the oral or nasal cavity, or indirectly from vomiting, into the trachea and lower respiratory system.
Terminology Issues
The HCS is currently somewhat inconsistent in the way the terms "hazard category" and "toxicity category" are used throughout appendix A. In some cases the terms are used interchangeably, while in other instances the terms are intended to have different meanings. OSHA has reviewed appendix A and is proposing revisions to ensure that these terms are used appropriately and consistently. As such, OSHA proposes to delete the term "toxicity category" and replace it with "hazard category" in various places, including paragraphs A.0.5, A.1, A.8, A.9, and A.10. These proposed changes would align with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2012, Document 0084).
Mandatory Language
OSHA is proposing to update a number of provisions in appendix A to make it clear that those provisions are mandatory. For example, OSHA proposes to change the term "should" to "must" in paragraph A.3.4.3.3. The change would clarify that the cut-off value/concentrations in Table A.3.3 are mandatory when determining if a mixture must be classified as seriously damaging to the eye or an eye irritant.
Corrections
OSHA proposes to correct a few errors that currently exist in the HCS. OSHA erroneously did not include appendix A.4, respiratory or skin sensitization, in the list of health hazards referenced in the "concentration of mixtures" paragraph at A.0.5.1.3. OSHA proposes Start Printed Page 9706 to add a reference to appendix A.4 in paragraph A.0.5.1.3 to clarify that the concentration of mixtures bridging principle applies to respiratory and skin sensitization. Similarly, appendix A.4 was also erroneously excluded from the list of health hazards referenced in the "interpolation within one toxicity category" paragraph at A.0.5.1.4. Thus, OSHA also proposes to add a reference to appendix A.4 in paragraph A.0.5.1.4 to clarify that the interpolation bridging principle applies to respiratory and skin sensitization. In addition, OSHA proposes to correct the cross-reference from A.1.3.6.2.3 to A.1.3.6.2.4 in Figure A.1.1 and paragraph A.1.3.6.2.2.
D. Appendix B
OSHA is proposing a number of changes to appendix B. First, since the HCS was aligned with the GHS in 2012, new physical hazard classes or hazard categories have been added to the GHS Rev. 7 (UN GHS, 2017, Document ID 0060). OSHA proposes to adopt those additions. Second, the GHS Rev. 7 (UN GHS, 2017, Document ID 0060) contains several updates to consensus standards and testing methods. Although the HCS does not require testing and permits classifiers to use data from literature or experience for classification purposes, OSHA is proposing to update consensus standards and testing methods referenced in appendix B in accordance with the GHS Rev. 7 to ensure that data considered for classification incorporate updated scientific principles. Third, OSHA is proposing certain corrections and clarifications to appendix B to address (1) previous inadvertent omissions from the GHS or the HCS; (2) changes made to the GHS to improve clarity or technical accuracy; and (3) how some hazard classes should be evaluated in light of the addition of new hazard classes in the GHS. These proposed changes are discussed below and would align the HCS with the GHS while improving the classification and communication of hazards[59] and maintaining or enhancing worker safety and health. Additionally, as noted elsewhere, aligning the HCS with the GHS would ease compliance burdens for U.S. stakeholders that must also comply with international requirements for hazard classification and communication.
OSHA is also proposing to make a limited number of changes to appendix B that arise out of its implementation of the HCS, as updated in 2012. These changes, explained below, would clarify compliance requirements. OSHA believes that all of these proposed changes maintain existing safety and health protections while easing or having no effect on the compliance burdens for regulated entities.
Finally, OSHA explains below that it is not proposing to incorporate one update reflected in the GHS Rev. 7 because that particular update is inconsistent with the scope of the HCS.
OSHA is providing a redline strike out version of appendix B, which reflects all of OSHA's proposed revisions, in the docket and on the OSHA website (OSHA, 2020, Document ID 0222; https://www.osha.gov/dsg/hazcom). This will allow interested parties to view all of the proposed changes in context. OSHA strongly encourages stakeholders to review that document in conjunction with the discussion of the proposed revisions below, as the discussion below does not fully describe all of the non-substantive or editorial changes OSHA is proposing.
Explosives—(Appendix B.1)
OSHA is proposing a few minor amendments to appendix B.1, Explosives. The first change that OSHA is proposing involves a clarification to the classification criteria for Division 1.6 explosives in B.1.2(f). Under the GHS Rev. 3, one of the criteria for classification of an article [OSHA uses the term "item" in the HCS] as a Division 1.6 explosive is that it contains "only" extremely insensitive detonating chemicals (UN GHS, 2009, Document ID 0085). The GHS Rev. 7 (UN GHS, 2017, Document ID 0060) states that the criteria is met if the article ["item" in the HCS] "predominantly" contains extremely insensitive detonating chemicals. OSHA is proposing to make the same change to paragraph B.1.2(f) of appendix B in the HCS. Changing the criteria from containing "only" extremely insensitive detonating chemicals to "predominantly" containing extremely insensitive detonating chemicals is more technically accurate and better aligns with the guidance in test series 7 in the UN Manual of Tests and Criteria (UN TDG, 2016, Document ID 0151). OSHA believes that consistency in the use of terms will reduce confusion for chemical manufacturers or importers when classifying explosives.
OSHA is also proposing to add two notes from the GHS (UN GHS, 2017, Document ID 0060) to appendix B, paragraph B.1.3.1, that are related to the addition of the desensitized explosives hazards class (proposed appendix B.17), which is discussed later in this document. The first new note OSHA is proposing to add (Note 2) would provide that explosives for which explosive properties have been suppressed or reduced must be classified as desensitized explosives. The second new note OSHA proposes (Note 3) would provide that some chemicals that are exempt from classification as explosives under UN Recommendations on the Transport of Dangerous Goods guidelines still have explosive properties, which must be communicated in section 2 (Hazard identification) and section 9 (Physical and chemical properties) of the SDS, as appropriate. The notes would be incorporated in the HCS with edits to change these provisions from recommendations in the GHS to requirements in the HCS (e.g., "may be a candidate for classification as" in the GHS would be revised to "shall be classified as" in the HCS) and to revise the GHS terminology to terminology more appropriate for the HCS (e.g., "substances and mixtures" in the GHS would be revised to. "chemicals" in the HCS).
Flammable Gases—(Appendix B.2.)
OSHA is proposing several changes to the Flammable Gases hazard class (appendix B.2). Most significantly, OSHA is proposing to subdivide Category 1 of this class into two subcategories, 1A and 1B, and to specify that pyrophoric gases and chemically unstable gases are to be classified as Category 1A. These proposed changes would provide more detailed information about the flammable gas hazards and track changes made in the GHS Rev. 7 (UN GHS, 2017, Document ID 0060) since OSHA updated the HCS in 2012. These proposed changes would allow downstream users to have a better understanding of the severity of the hazards associated with flammable gases. Downstream users could then use this information to take appropriate precautions or determine if a substitute chemical is less hazardous.
The HCS currently lists two categories for flammable gases—Category 1 (Extremely flammable) and Category 2 (flammable)—that are based on the percentage of the gas in a mixture with air that is ignitable and on ranges of flammability in air. In practice, with the current criteria, almost all flammable gases (except ammonia and methyl bromide, which are treated separately) are classified as Category 1. This means that, for hazard identification and communication purposes, no distinctions are being drawn between gases that exhibit a wide spectrum of flammable properties. OSHA has preliminarily concluded that Category 1 Start Printed Page 9707 is too broad and can lead downstream users to choose a chemical without realizing that an alternative choice is actually less flammable. For example, 2,3,3,3-Tetrafluoropropene is a non-ozone depleting refrigerant which ignites less rapidly or violently than some other flammable gases. Many of these types of gases were developed as a result of the Montreal and Kyoto protocols, international treaties intended to phase out gases that are ozone depleting (UN GHS, 2016, Document ID 0138). However, with the current classification system, propane, which has a rapid, explosive ignition with a burn velocity of 46 cm/s, and 2,3,3,3-Tetrafluoropropene (R-1234yf), which has a slow, weak ignition, with a burn velocity of 1.5 cm/s, would both be classified as Category 1 gases, thus making it appear that the two gases are equally flammable when in fact 2,3,3,3-Tetrafluoropropene is considerably less flammable (UN GHS, 2016, Document ID 0138).
OSHA and DOT actively participated in the UN negotiations (joint informal working group) in 2015 to ensure that flammable gases are properly evaluated, classified and communicated. The joint informal working group activities included identifying, gathering, and reviewing data on "less flammable" gases, including the conduct of numerous burning velocity tests using approved test methods, as well as tests to demonstrate ignition behavior, flame propagation, and the speed of the flame front (UN GHS, 2016, Document ID 0254).
The revised classification criteria in Table 2.2.1 in Chapter 2.2 of the GHS Rev. 7 (UN GHS, 2017, Document ID 0060) reflect recommendations made by the joint informal working group. The joint informal working group agreed that all flammable gases currently classified as Category 1 flammable gases should remain so. This decision allows the transport classification and communication scheme to remain the same. However, the joint informal working group agreed that Category 1 should be separated into two sub-categories, Category 1A and Category 1B, when data is available on burning velocity and lower flammability limit. This separation allows for more precise classification of chemicals and more appropriate communication of the hazards associated with flammable gases.
This proposed approach for classifying flammable gases is also consistent with the approach described in ANSI/ASHRAE Standard 34-2013—Designation and Safety Classification of Refrigerants (ANSI/ASHRAE, 2013, Document ID 0160). The ANSI/ASHRAE standard allows refrigerant gases (which can be category 1A or 1B) to be classified based on both the lower flammability limit and burning velocity (see Figure 6.1.4 and Section 6.1.3.2.1 (ANSI/ASHRAE, 2013, Document ID 0160). OSHA's proposed cut-off for the burning velocity for category 1A and 1B chemicals is the same as that in the ASHRAE standard. Therefore, the proposed approach is consistent with accepted scientific principles and industry norms.
OSHA has preliminary concluded that the classification scheme in Table 2.2.1 of the GHS Rev. 7 (UN GHS, 2017, Document ID 0060) maintains safety for workers while allowing for more precise hazard classification and communication. Therefore, OSHA is proposing to replace Table B.2.1 of the HCS with the criteria from Table 2.2.1 in the GHS Rev. 7 (UN GHS, 2017, Document ID 0060). The only modification that OSHA proposes making to the GHS Table 2.2.1 is to add units of measurement used in the United States (e.g., degrees Fahrenheit). Under the proposed new table, all flammable gases that are currently classified as Category 1 flammable gases would be classified as Category 1A, unless data on flammability limit or fundamental burning velocity indicates that the gas should be classified as Category 1B. For a gas to be classified in Category 1B, data would have to show that its lower flammability limit is more than 6% by volume in air or its fundamental burning velocity is less than 10 cm/s; in addition, the gas could not be either pyrophoric or chemically unstable. Since the HCS does not require testing, the data required to classify a gas as a Category 1B flammable gas could be obtained from literature. However, if data is lacking in the literature, then testing would be necessary to establish that a newly-developed flammable gas qualifies for classification as a Category 1B flammable gas. The joint informal working group compiled a list of data available on burning velocity and flammability limits for pure flammable gases (OSHA, 2017, Document ID 0164).
When OSHA revised the HCS in 2012, pyrophoric gases were not classified under the GHS, Rev. 3 (UN GHS, 2009, Document ID 0085). Therefore, to ensure that the hazards of pyrophoric gases would continue to be covered and communicated, OSHA maintained the approach taken in the HCS starting in 1994. This involved addressing pyrophoric gases under the definition of "hazardous chemical" and maintaining a definition for "pyrophoric gas" in paragraph (c) of the HCS (77 FR 17704). While OSHA retained the definition for "pyrophoric gas" when it updated the HCS in 2012, the agency explained it also intended to continue to work with the UNSCEGHS to add the pyrophoric gas hazard to the GHS, along with two other hazards that OSHA covered under the HCS but that were not classified under the GHS: Simple asphyxiants and combustible dust (77 FR 17704). Since OSHA revised the HCS in 2012, the UNSCEGHS updated the criteria for flammable gases to include pyrophoric gases (UN GHS, 2014, Document ID 0086; UN GHS, 2017, Document ID 0060). The UNSCEGHS agreed that pyrophoric gases, as well as chemically unstable gases, should always be classified as Category 1A flammable gases because of the nature of these two types of gases; pyrophoric gases ignite spontaneously in air at temperatures of 54 °C (130 °F) or below, and chemically unstable gases are able to react explosively even in the absence of air or oxygen. Under the GHS Rev. 7 (UN GHS, 2017, Document ID 0060), pyrophoric gases and chemically unstable gases are both classified as Category 1A. OSHA preliminarily agrees with this decision and proposes incorporating it into appendix B.2.
If, as proposed, OSHA adds pyrophoric gases as a sub-category of flammable gases in appendix B.2, and, as proposed, includes a definition of pyrophoric gas in appendix B.2., it would no longer be necessary to include these gases as part of the definition of "hazardous chemical" or to include a definition for "pyrophoric gas" in § 1910.1200(c). Therefore, OSHA proposes to delete those terms in § 1910.1200(c). OSHA also proposes to incorporate the definition of "pyrophoric gas" found in the GHS Rev. 7 (UN GHS, 2017, Document ID 0060), in paragraph B.2.1. OSHA currently defines pyrophoric gas as a chemical in the gaseous state that "will ignite" spontaneously in air at a temperature of 130 °F (54.4 °C) or below. The GHS Rev. 7 defines a pyrophoric gas as a flammable gas that is "liable to ignite" spontaneously in air at a temperature of 54 °C (130 °F) or below (UN GHS, 2017, Document ID 0060). The change in the definition from a gas that "will ignite" to a gas that is "liable to ignite" was made because some pyrophoric gases may have a delayed ignition time (UN GHS, 2013, Document ID 0086). OSHA preliminarily finds the term "liable to ignite" to be more accurate, from a technical perspective. OSHA does not believe that these changes would have a significant impact on the scope of Start Printed Page 9708 gases considered pyrophoric gases, nor does OSHA expect that chemical manufacturers or importers would need to reclassify chemicals due to these changes.
As noted above, OSHA proposes adding a new sub-category for chemically unstable gases to the flammable gases hazard class to allow for more accurate communication of the hazards associated with those gases. OSHA proposes to adopt the GHS Rev. 7 definition of a chemically unstable gas, i.e., a flammable gas that is able to react explosively even in the absence of air or oxygen (UN GHS, 2017, Document ID 0060), in paragraph B.2.1. Consistent with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060), under proposed Table B.2.1, a Category 1A chemically unstable gas would be further sub-characterized into one of two categories based on the temperature and pressure at which it becomes unstable. The proposed criteria for Category 1A/A chemically unstable gases are flammable gases which are chemically unstable at 20 °C (680 °F) and a standard pressure of 101.3 kPa (14.7 psi). The proposed criteria for Category 1A/B chemically unstable gases are flammable gases which are chemically unstable at a temperature greater than 20 °C (680 °F) and/or a pressure greater than 101.3 kPa (14.7 psi).
As chemically unstable gas is a subcategory of flammable gases, any chemical that meets the criteria for chemically unstable gas meets the current definition of flammable gas. While these hazards are currently classified in flammable gases under the HCS the UNSCEGHS noted that these gases exhibit slightly different behaviors and have the propensity to react dangerously even in the absence of any reaction partner (e.g., air or oxygen) and should have different hazard communication elements (UN GHS, 2010, Document ID 0210). Types of flammable gases or gas mixtures that might be candidates for classification as chemically unstable are flammable gases with functional groups such as triple-bonds, adjacent or conjugated double-bonds, halogenated double-bonds, and strained rings (UN GHS, 2010, Document ID 0210). Because chemical manufacturers are currently classifying chemically unstable gases as flammable gases, OSHA does not consider these gases to be a new hazard. Instead, OSHA believes the addition of chemically unstable gases as a separate category in the appendix for flammable gases (appendix B.2) would improve the way the hazards of these gases are identified, evaluated, and communicated.
The GHS Rev. 7 (UN GHS, 2017, Document ID 0060) added three clarifying notes under Table 2.2.1 that were not included in the GHS Rev. 3 (UN GHS, 2009, Document ID 0085). The notes provide guidance on the classification of flammable gases under the new hazard categories. OSHA is proposing to add these notes to the HCS following Table B.2.1 (as new Note 2, Note 3, and Note 4) because they allow for better hazard classification.
The GHS Rev. 7, in Chapter 2.2.4.2, provides additional guidance on the classification of flammable gases, including the new hazard categories of pyrophoric gases, chemically unstable gases, and 1B flammable gases (UN GHS, 2017, Document ID 0060). It includes updated references to consensus standards and test methods (i.e., ISO 10156:2010), and new references to consensus standards and test methods related to the new hazard categories (i.e., ISO 817:2014, IEC 60079-20-1 ed1.0 (2010-01), or DIN 51794, and Part III of UN of the Manual of Tests and Criteria). OSHA proposes to adopt these changes in the HCS appendix B.2.3, with edits to make the GHS criteria mandatory (i.e., changing "should" to "shall"), to add U.S. units of measurement (e.g., Fahrenheit), and to add statements that cited standards and test methods are incorporated by reference under 29 CFR 1910.6. This proposed modification would also align with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060). However, OSHA does not intend to require those already classified using an earlier version of ISO 10156, only those classifying new chemicals or chemicals not already classified. To incorporate this guidance from the GHS Chapter 2.2.4.2, OSHA is proposing edits to existing paragraph B.2.3. (B.2.3.1, as proposed) and new paragraphs B.2.3.2, B.2.3.3, and B.2.3.4.
Aerosols—(Appendix B.3)
OSHA is proposing to follow the GHS Rev. 7 (UN GHS, 2017, Document ID 0060) by expanding the existing Flammable Aerosols hazard class (appendix B.3) to include non-flammable aerosols as well as flammable ones. Under the GHS Rev. 3 and the current HCS, Chapter 2.3 and appendix B.3, respectively, were titled "Flammable Aerosols." Under the GHS Rev. 3, the hazards presented by non-flammable aerosols were either not classified at all or, more likely, were classified in another health hazard class or physical hazard class (e.g., gases under pressure) (UN GHS, 2009, Document ID 0085). Flammable aerosols were likely to be classified as both flammable aerosols and gases under pressure.
OSHA believes that most aerosols are classified as gases under pressure under the GHS Rev. 3 (and accordingly under the existing HCS) because of the design criteria of the aerosols (ERG, 2015, Document ID 0163) under DOT regulations. Under DOT regulations, aerosols are non-refillable receptacles containing a gas compressed, liquefied, or dissolved under pressure, and the highest permissible pressure is 180 psig at 130 °F (see 49 CFR 171.8, 173.306). Accordingly, under DOT regulations, most aerosols meet the current HCS criteria for gases under pressure, which are gases contained in a receptacle at a pressure of 200 kPa (29 psi) or more, or which are liquefied or liquefied and refrigerated (see existing paragraph B.5.1 in appendix B.5). However, OSHA believes that classifying aerosols as gases under pressure may not accurately identify the hazards of aerosols because aerosol containers differ from pressurized gas cylinders in terms of container characteristics and failure mechanisms, as described further below.
Since the GHS Rev. 3 (UN GHS, 2009, Document ID 0085), the UNSCEGHS and the UN Sub-committee of Experts on the Transport of Dangerous Goods (UNSCETDG) agreed to rename Chapter 2.3 "Aerosols" and to add a new non-flammable aerosol hazard category, Category 3, to the aerosols hazard class (UN GHS, 2018, Document ID 0249). This hazard category captures aerosols that (1) contain 1% or less flammable components (by mass); and (2) have a heat of combustion that is less than 20 kJ/g.
Before proposing to adopt this category for non-flammable aerosols, OSHA reviewed the impact of this change to ensure that it would not compromise worker safety and health. OSHA assessed the hazards associated with aerosol containers and compressed gas cylinders. An ERG study evaluated how aerosol products and gases under pressure differ in terms of container characteristics, failure mechanisms, and previous incidents (ERG, 2015, Document ID 0009).
The ERG report concluded that sizes and pressures of compressed gas cylinders far exceed those of hand-held containers typically used for aerosol products (ERG, 2015, Document ID 0009). The report also noted differences in failure mechanisms for pressurized cylinders versus aerosols (ERG, 2015, Document ID 0009). As an example, increased temperatures can result in the release of container contents from the activation of pressure relief devices on Start Printed Page 9709 cylinders, whereas increased temperatures can result in the bursting of aerosol cans, which do not contain pressure relief devices. Also, hazards from falling cylinders include the release of contents following the valve breaking, the cylinder becoming a projectile or pinwheel, or the crushing of employees in the area; although aerosol containers can be damaged if they are dropped or punctured, they do not pose the same hazards as falling cylinders.
ERG reported that occupational incidents involving cylinders included explosions during high temperature activities (such as welding) and explosions resulting from mechanical deformation (e.g., from falling cylinders), over-pressurization of cylinders (e.g., from overfilling, which can result in a rupture of the cylinder), or leaks due to corrosion (ERG, 2015, Document ID 0009). Most incidents with aerosol cans involved explosions following heating or puncture of the can (ERG, 2015, Document ID 0009). The ERG report concluded that although non-flammable aerosol cans do not present a significant fire hazard, they can present a hazard from bursting resulting from thermal content expansion during heating. (ERG, 2015, Document ID 0009).
In addition to the ERG report, OSHA also considered data from the agency's Fatality and Catastrophe Information Summary (FatCat) database, located at https://www.osha.gov/pls/imis/accidentsearch.html (Document ID 0204), to evaluate the nature and severity of injuries and fatalities resulting from the use of aerosols and compressed gases. To determine if an incident was related to aerosols or compressed gas cylinders, OSHA searched for the keywords "aerosol," "spray," or "foam" (to identify aerosols), and the keywords "compressed," "cylinder," or "CNG" (to identify compressed gases). The data reviewed is available in the docket (OSHA, 2019, Document ID 0204).
From 1995 to 2014 there were more incidents related to the use of compressed gas cylinders than to the use of aerosol containers, but the percentage of the incidents that resulted in fatalities was similar (29% versus 28%, respectively). However, as explained below, fatalities are more likely to be associated with the container itself when compressed gas receptacles are in use as compared to situations involving aerosol containers. (OSHA, 2019, Document 0204).
Fatalities associated with use of compressed gas cylinders and aerosol containers primarily fall into three categories: (1) Incidents due to the contents of the container, such as flash fires or explosions; (2) incidents due to the container itself, such as incidents related to pressure, container failure, or ruptures; and (3) incidents unrelated to the use of the container, such as heart attacks or falls. A higher percentage of fatalities fell into the second category (incidents related to the container itself) for compressed gas cylinders (64% of the compressed gas cylinder fatalities) than for aerosol containers (17% of the aerosol fatalities). Conversely, a greater proportion of fatalities related to aerosols were attributed to reasons other than the container itself (83% for aerosol containers versus 36% for cylinders) (OSHA, 2019, Document ID 0204). This included fatalities related to the contents of the container and those in the third, "miscellaneous," category (where the fatality could not be directly related to the use of the container, e.g., situations such as heart attacks, falls, lack of training that occurred while employees were working with, or that generally related to, the use of aerosol or compressed gas cylinders) (OSHA, 2019, Document 0204). Thus, it appears that employees are at greater risk of a fatality due to the failure of the container if they are working with compressed gas cylinders than they are if they are working with aerosol cans.
Following a review of the data and the ERG report, OSHA preliminarily concludes that a new category for non-flammable aerosols is appropriate. OSHA believes this category would allow the hazards of non-flammable aerosols to be more appropriately classified and communicated, resulting in improved worker protection. The new hazard category would provide downstream users with more appropriate communication on the label by adding precautionary statements: Keep away from heat, hot surfaces, sparks, open flames and other ignition sources; No smoking; and Do not pierce or burn, even after use (see appendix C). Additionally, this new classification would ensure compressed gas pictograms are not included on aerosol labels, eliminating the risk of "over warning" about the hazards of aerosol containers (UN, 2010, Document ID 0095).
Specific changes OSHA is proposing include: Updating the aerosol hazard class to include non-flammable aerosols (hazard Category 3 in Table B.3.1), changing the name of appendix B.3 from "Flammable Aerosols" to "Aerosols," replacing the phrase "flammable aerosols" with "aerosols" throughout appendix B.3, as appropriate, and adding clarifying information from the GHS Rev. 7 to paragraph B.3.2 (UN GHS, 2017, Document ID 0060). For example, OSHA is proposing to revise Note 2 to B.3.2.1 to explain that aerosols do not fall within the scope of gases under pressure, but may fall within the scope of other hazard classes. OSHA's preliminarily conclusion that aerosols (flammable and non-flammable) should not also be classified as gases under pressure would ensure that the appropriate hazard warnings are presented on aerosol containers.
OSHA is proposing to adopt the GHS Rev. 7 (UN GHS, 2017, Document ID 0060) criteria for a non-flammable aerosol (i.e., an aerosol that does not meet the criteria for Category 1 or 2, contains less than or equal to 1 percent flammable components (by mass), and has a heat of combustion less than 20 kJ/g), and to add those criteria as new Category 3 in Table B.3.1. This new category, Category 3, would update hazard communication requirements to better reflect the true hazards of non-flammable aerosols. This would result in changing the labeling for any such aerosols that are currently classified as compressed gases. In these situations, the "gas cylinder" pictogram would become unnecessary, as this hazard class would no longer be considered a compressed gas, the signal word "danger" would change to "warning," due to the decreased hazard, and a hazard statement of "contains gas under pressure; may explode if heated" would change to "pressurized container, may burst if heated", which would more accurately reflect the hazards associated with this category (see proposed appendix C.4.16). As discussed above, OSHA believes that this approach would better differentiate between the hazards associated with compressed gases and the hazards associated with aerosols.
Oxidizing Gases—(Appendix B.4)
OSHA proposes to revise the note in B.4.1, and the text in the "Additional classification considerations" paragraph at B.4.3, to clarify that the provisions are referring to the most recent version of the ISO 10156 standard, (ISO, 10156, 2010). This proposed change would provide more clarity on the definition and classification of oxidizing gases and lead to more accurate classification and improved communication. This proposed modification would also align with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060). However, OSHA does not intend to require those already classified using an earlier version of ISO 10156, only those classifying new chemicals or chemicals not already classified. Start Printed Page 9710
Gases Under Pressure—(Appendix B.5)
OSHA is proposing to change the definition of gases under pressure in B.5.1 to align with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060). OSHA proposes to add a temperature of 20 degrees Celsius (68 degrees Fahrenheit) to the definition so that the full definition would define gases under pressure as gases which are contained in a receptacle at a pressure of 200 kPa (29 psi) (gauge) or more at 20 °C (680 °F), or which are liquefied or liquefied and refrigerated. The change is intended to clarify that the pressure of the receptacle is measured at standard conditions. OSHA is also proposing to add a note to Table B.5.1 to clarify that aerosols should not be classified as gases under pressure. This proposed change is a consequence of OSHA's proposal to add a new hazard category for non-flammable aerosols, as discussed previously.
Flammable Liquids—(Appendix B.6)
OSHA is proposing to make three clarifying changes to the flammable liquid hazard class in appendix B.6. First, OSHA is proposing to add a reference to the Flammable Liquids standard, specifically 29 CFR 1910.106(a)(14), in paragraph B.6.3 in order to provide additional guidance about methods that can be used to determine flashpoint.
Second, after updating the HCS in 2012, OSHA realized there may be a concern with ensuring that information needed to determine the appropriate storage for flammable liquids is adequately documented on the SDS. Per 29 CFR 1910.106(a)(5), when an accurate boiling point is unavailable, or for mixtures which do not have a constant boiling point, the boiling point may be based on the 10% point of a distillation performed in accordance with the Standard Method of Test for Distillation of Petroleum Products, ASTM D-86-62. Together with an appropriately measured flash point, this boiling point can be used to categorize the mixture for use with Table H-12 in § 1910.106 to determine the maximum allowable container size and type. Use of a boiling point reported in section 9 of an SDS (physical properties), which is based on the "first drop" (or initial) distillation temperature in D-86, will likely be conservative, but may lead to more restrictive storage requirements than would be the case using the 10% distillation point (see appendix D, section 9(f)). OSHA is proposing to add a clarifying footnote to B.6.3 explaining that to determine the appropriate container size and container type for a flammable liquid, the boiling point must be determined by the methods specified under OSHA's Flammable Liquids standard (29 CFR 1910.106(a)(5)) and listed on the SDS. In addition, the proposed note would explain that the chemical manufacturer, importer, or distributor must clearly note on the SDS (in sections 7 and 9) if a calculation other than initial boiling point was used for storage purposes. OSHA did not intend for the updated HCS classification requirements for flammable liquids to impact the longstanding storage requirements under 29 CFR 1910.106 and views this proposed note as a method to ensure that the proper container size and type will be used for storing flammable liquids and that all necessary information is appropriately communicated on the SDS. OSHA is not proposing any changes to the classification criteria for flammable liquids under the HCS. OSHA is requesting comments on whether a footnote like the one proposed for B.6.3 should also be inserted in appendix D, section 9.
Finally, OSHA realized that a note regarding cross-classification of aerosols was inadvertently omitted from appendix B.6 (flammable liquids). In appendix B.3 (flammable aerosols), note 2 to the classification criteria currently indicates that "[f]lammable aerosols do not fall additionally within the scope of flammable gases, flammable liquids, or flammable solids." The HCS currently contains a cross-referencing note in appendix B.2 (flammable gases), but OSHA inadvertently omitted the statement in appendix B.6 (flammable liquids). OSHA is therefore proposing to add a note stating that aerosols should not be classified as flammable liquids in appendix B.6, following Table B.6.1, for consistency and to minimize confusion. This would align with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060).
Flammable Solids—(Appendix B.7)
The only change proposed to appendix B. 7 (flammable solids) is a new note (Note 2) following Table B.7.1 stating that aerosols should not be classified as flammable solids. As with flammable liquids, the UNSCEGHS observed this omission in the flammable solids chapter, and the GHS Rev. 7 includes this note (UN GHS, 2017, Document ID 0060).
Self-Heating Chemicals—(Appendix B.11)
OSHA proposes adding a note to Table B.11.1. This proposed note would explain that classification of solid chemicals shall be based on tests performed on the chemical as presented. For example, if the chemical is presented for supply or transport in a physical form different from that which was tested and which is considered likely to materially alter its performance in a classification test, classification must be based on testing of the chemical in the new form. Although this note was included in the GHS Rev. 3 (UN GHS, 2009, Document ID 0085), and incorporated into appendices B.1, B.7, B.10, B.12 and B.14 in the HCS in 2012, it was inadvertently omitted from appendix B.11. OSHA is proposing to add the note to be consistent with the GHS and the way the HCS treats other physical hazards.
Chemicals Which, in Contact With Water, Emit Flammable Gases—(Appendix B.12)
OSHA proposes to update the classification criteria for Category 3 of this hazard class in Table B.12.1. In the GHS Rev. 3 (UN GHS, 2009, Document ID 0085) and the existing HCS, one of the criteria for a Category 3 classification is that the maximum rate of evolution of the flammable gas is "equal to or greater than 1 liter per kilogram of chemical per hour." However, this criteria does not accurately reflect the corresponding criteria in Test N.5 (test method for substances which, in contact with water, emit flammable gases) in Part III, sub-section 33.4.1.4.4.4 of the UN Manual of Tests and Criteria (UN TDG, 2016, Document ID 0151), which provides that the maximum rate of evolution of the flammable gas is greater than 1 liter per kilogram of chemical per hour. OSHA proposes to delete the words "equal to or" in the Category 3 criteria in Table B.12.1 to make the classification criteria consistent with the criteria in the test method. This will align the HCS with the GHS Rev.7 (UN GHS, 2017, Document ID 0060) and would not affect worker protections.
Oxidizing Solids—(Appendix B.14)
OSHA is proposing to add a second set of classification criteria to B.14.2 and to Table B.14.1.based on a new UN test method. Under the GHS Rev. 3 (UN GHS, 2009, Document, ID 0085), classification of oxidizing solids was based only on Test O.1 from Part III, sub-section 34.4.1 of the UN Manual of Tests and Criteria (UN TDG, 2016, Document ID 0151). This is reflected in the current HCS, appendix B.14. However, the test material used as the reference mixture in Test O.1 has been noted to pose a cancer hazard and is difficult to purchase. Therefore, a new test, Test O.3, Gravimetric tests for Start Printed Page 9711 oxidizing solids, has been added to Part III, sub-section 34.4.3 of the UN Manual of Tests and Criteria (UN TDG MTC, 2016, Document ID 0151). This new test underwent a thorough evaluation, including round robin testing, led by the UNSCETDG (UN SCETDG, 2016, Document ID 0150). Test O.3 uses a reference mixture of calcium peroxide and cellulose, whereas Test O.1 uses the reference substances potassium bromate and cellulose (UN TDG, 2016, Document ID 0165).
Consistent with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060), OSHA proposes to allow oxidizing solids to be classified using either Test O.1 or Test O.3. Since the proposed classification criteria would allow the use of data from either Test O.1 or O.3, data from existing classifications could be used and no new testing would be required for substances or mixtures that were previously classified based on Test O.1.
OSHA also proposes to update Note 1 to Table B.14.1 to reflect a 2017 revision to the International Maritime Solid Bulk Cargoes Code for testing of explosion hazards (IMSBC, 2017, Document ID 0141).
Corrosive to Metals—(Appendix B.16)
OSHA is not proposing to make any changes to appendix B.16, Corrosive to Metals. This is notable because OSHA has preliminarily decided not to adopt a note that was added in the GHS Rev. 7. Table 2.16.2 in Chapter 2.16 of the GHS Rev. 7 (UN GHS, 2017, Document ID 0060) contains a note stating: "Where a substance or mixture is classified as corrosive to metals but not corrosive to skin and/or eyes, some competent authorities may allow the labelling provisions described in 1.4.10.5.5." Chapter 1.4.10.5.5 contains labeling provisions that apply to "substances or mixtures which are in the finished state as packaged for consumer use." OSHA has preliminarily concluded that the note in question, and the labeling provisions it refers to, are not applicable to the HCS because the HCS applies only to use of chemicals in the workplace, and not to consumer products (see 29 CFR 1910.1200(b)(5)(v)). Therefore, OSHA is not proposing to adopt the note found in Table 2.16.2 of Chapter 2.16 of the GHS Rev. 7.
Desensitized Explosives—(Appendix B.17)
OSHA is proposing to follow the GHS Rev. 7 (UN GHS, 2017, Document ID 0060) by adding a new physical hazard class for desensitized explosives. Desensitized explosives are chemicals that are treated in such a way to stabilize the chemical or reduce or suppress its explosive properties. These types of chemicals can pose a hazard in the workplace when the stabilizer is removed, either as part of the normal work process or during storage of the chemical. Therefore, it is important that the hazards be identified and appropriately communicated.
In the HCS, as revised in 2012, OSHA acknowledged, consistent with the GHS Rev. 3 (UN GHS, 2009, Document ID 0085), that these chemicals are considered explosives if the wetting agent is removed, by including the precautionary statement "keep wetted with" and instructing the chemical manufacturer, importer, or distributor to specify appropriate material for wetting if drying out increases the explosion hazard (see existing appendix C at C.4.14). However, the hazard statement, signal word, pictogram and other precautionary statements required under existing C.4.14 are geared to more conventional explosives. This gap in communication was recognized as early as 2005, when the UNSCEGHS noted that desensitized explosives may become explosive under certain circumstances—especially after long term storage and during handling and use (UN GHS, 2005, Document ID 0206). The UNSCEGHS examined the issue of hazard classification for desensitized explosives and concluded a new hazard class was warranted to ensure the appropriate hazard statement, signal word and precautionary statements for desensitized explosives were incorporated into the GHS (UN GHS, Report, 2014, Document ID 0087). The GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2016, Document ID 0142) separately classified desensitized explosives with a full set of unique label elements (including the appropriate signal word, hazard statement, pictogram, and precautionary statements). As separately classified, desensitized explosives are labeled with a flame pictogram rather than the explosive bomb used for explosives, and the precautionary statements are tailored to the specific traits of desensitized explosives (e.g., Avoid heating under confinement or reduction of the desensitizing agent.).
OSHA reviewed the UNSCEGHS reports (UN GHS, 2014, Document ID 0087) on desensitized explosives and has preliminarily concluded that the hazard class should also be added to the HCS to improve communication about these hazards. While the chemicals captured by the desensitized explosives hazard class are currently covered under the scope of the HCS as explosives, OSHA believes there is a benefit to providing classification criteria and corresponding hazard communication specific to this hazard. Adding the proposed new hazard class to the HCS would ensure downstream users receive more accurate hazard information on labels and in SDSs for these chemicals.
For these reasons, and to align with the GHS, OSHA proposes to add the desensitized explosives hazard class to the HCS as appendix B.17. Proposed appendix B.17 provides relevant definitions and general considerations, specifies applicable classification criteria, and includes information about additional classification considerations for this hazard class. It also references several sections from the UN Recommendations on the Transport of Dangerous Goods, Manual of Tests and Criteria that will be incorporated by reference. As with all hazard classes, the GHS Rev. 7 (UN GHS, 2017, Document ID 0060) does not require testing and allows classifiers to use data reported in the literature (UN TDG, 2016, Document ID 0151) that was generated using specified (or equivalent) test methods. Proposed appendix C.4.30, discussed later in this document, contains proposed communication elements for desensitized explosives.
Proposed appendix B.17 is based on Chapter 2.17 of the GHS Rev. 7 (UN GHS, 2017, Document ID 0060). OSHA is proposing to adopt most of the classification language on desensitized explosives from Chapter 2.17 of the GHS Rev. 7 (UN GHS, 2017, Document ID 0060) to minimize deviations from the GHS. However, OSHA has carefully reviewed each of the hazard classification criteria within the context of the HCS and is proposing to modify some of the language. These edits include changing some recommendations in the GHS to mandatory requirements in the HCS (i.e., changing "should" to "shall"); revising some terms in the GHS to more accurately reflect terminology in the HCS (e.g., changing "manufacturer/supplier" to "manufacturers, importers, and distributors"); revising text to make it clear that data for classification can be obtained from the literature; and removing references to classifications for transportation that do not apply under the HCS. Some of the GHS text stressing where a classification scheme is for scenarios other than transportation would also be removed (e.g., terms referring to storage, supply, and use); this change is being proposed because the HCS does not cover transportation, and it is therefore not necessary to include such language in appendix B of the HCS. OSHA also proposes adding a definition for "phlegmatized" in a Start Printed Page 9712 footnote because many stakeholders may be unfamiliar with that term from the UN Recommendations (UN GHS, 2017, Document ID 0060).
OSHA is not proposing to include portions of the GHS Chapter 2.17 that do not relate specifically to the method of classification for desensitized explosives; this is the same approach OSHA took in the 2012 update. For example, similar to how OSHA has addressed the other hazard classes, OSHA is not proposing to adopt the decision logics from the GHS in appendix B.17; OSHA may, however, use them in guidance materials. Also, OSHA did not include text relating to hazard communication in proposed appendix B.17 because this information is contained in proposed appendix C.
E. Appendix C
OSHA is proposing a number of updates to appendix C of the HCS in an effort to improve communication of hazard information on labels. These proposed changes will: (1) Address labeling requirements for the new hazard classes and categories in appendix B (physical hazards); (2) align the HCS with the GHS Rev. 7; and (3) improve alignment of the HCS and Health Canada's labeling requirements in furtherance of the goals of the RCC.
Appendix C is the mandatory appendix that includes the requirements and instructions for the allocation of label elements. Paragraph (f)(2) of the HCS requires the chemical manufacturer, importer, or distributor to ensure that the information provided on the label is in accordance with appendix C. Appendix C provides hazard statements, signal words, pictograms, and precautionary statements for all four essential aspects of hazardous chemical management (prevention, response, storage and disposal), as well as general labeling instructions.
As discussed in the 2009 NPRM proposing to align the HCS with the GHS, the precautionary statements, unlike the hazard statements, were not harmonized (but were merely codified) under the GHS, meaning that numbers were assigned to them. This meant that the statements were not yet considered to be part of the harmonized text (like hazard statements); rather they were included in the GHS as suggested language (74 FR 50282-83). OSHA chose to add these statements in the final HCS rule in 2012 (77 FR 17574). However, since the promulgation of the updates to the HCS in 2012, the UNSCEGHS has continued work to improve the utility of precautionary statements by providing better guidance on the allocation of statements, updating the statements to provide better protection, and adding new statements for new hazard classes and categories. OSHA is proposing a number of changes based on new precautionary statements and instructions in the GHS Rev. 7. Additionally, since 2012, OSHA has continued to work with other Federal agencies on crosscutting labeling issues. The updates proposed in appendix C would ensure alignment with DOT labeling regulations and are expected to provide the same level of protection for workers as the current HCS. OSHA is also proposing updates to appendix C based on the agency's cooperation with Health Canada under the RCC. The RCC was reaffirmed through a memorandum of understanding that was signed in June 2018 (RCC, 2019, Document ID 0217), with the expectation of aligning efforts for international trade requirements between the two countries.
Overall, OSHA expects that the proposed changes to appendix C would provide improved safety information and greater detail and clarity for downstream users. They also would provide better consistency that bridges the jurisdictional differences between countries and Federal agencies. Aligning the HCS with the GHS and other Federal or international regulations would ease compliance burdens for U.S. stakeholders that must also comply with those requirements. The changes that OSHA is proposing would lead to improved communication of hazard information, which would maintain or enhance the safety and health of workers.
The changes OSHA is proposing to appendix Care extensive. OSHA addresses the substantive proposed changes in the discussion below, and a redline strike out version of appendix C, which reflects all of OSHA's proposed revisions, is available in the docket and on the OSHA website (OSHA HCS Redline Document, 2020, Document ID 0222; https://www.osha.gov/dsg/hazcom/). This will allow interested parties to view all of the proposed changes in context. OSHA strongly encourages stakeholders to review that document in conjunction with the discussion of the proposed revisions below, as the discussion below does not fully describe all of the non-substantive or editorial changes OSHA is proposing.
Proposed Changes to C.1-C.3
The instructions currently found in the beginning of appendix C (see C.1-C.3) provide directions and information about the signal words, pictograms, hazard statements and precautionary statements required per C.4. OSHA is proposing changes to C.1-C.3 to align with the GHS Rev. 7, better harmonize the HCS with DOT regulations, and better harmonize the HCS with Health Canada.
First, OSHA proposes to revise Figure C.1—Hazard Symbols and Classes to include "HNOC (non-mandatory)" as a hazard identified by the exclamation point pictogram. This proposed change reflects OSHA's agreement with Health Canada to permit the exclamation mark pictogram to be used for HNOCs. While OSHA does not require labelling for HNOC hazards, Health Canada requires a pictogram, signal word, hazard statements, and precautionary statements for HNOCs. In order to ensure that U.S. and Canadian requirements can simultaneously be met for HNOCs, OSHA and Health Canada have provided guidance allowing an exclamation mark pictogram to be used for HNOCs (OSHA, 2016, Document ID 0103). Use of the exclamation mark pictogram would not be mandatory under the HCS.
Relatedly, OSHA is proposing a number of additional changes. As discussed above, OSHA is proposing to move the current C.2.3.3 from appendix C to paragraph (f)(5) in the text of the standard, so that all of the instructions related to the transport of hazardous chemicals and DOT are in one section of the HCS. OSHA is also proposing to add a new paragraph C.2.3.3, which would allow the exclamation mark pictogram to be used for HNOCs if the words "Hazard Not Otherwise Classified" or the letters "HNOC" appear below the pictogram on the label. Health Canada and OSHA have agreed that the exclamation mark pictogram is an appropriate symbol for the HNOC, HHNOC (Health Hazards Not Otherwise Classified), and PHNOC (Physical Hazards Not Otherwise Classified) classifications. Additionally, because any pictogram may appear only once on a label, OSHA is also proposing to add a new paragraph at C.2.3.4 to specify that if multiple hazards require use of the same pictogram, it may not appear a second time on the label. This includes when the exclamation mark pictogram would be used, including as supplemental information for another hazard, such as HNOC. OSHA is requesting comments on these proposed changes, and is particularly interested in comments on whether the agency should require the exclamation mark pictogram to be used for HNOCs.
The remaining changes proposed for C.2 reflect updates to the GHS that are intended to provide additional flexibility to the label preparer while still communicating the required Start Printed Page 9713 information. OSHA is proposing to add new paragraph C.2.4.7 to note that precautionary statements may contain minor textual variations from the text prescribed elsewhere in appendix C (e.g., spelling variations, synonyms or other equivalent terms), as long as those variations assist in the communication of safety information without diluting or compromising the safety advice. This proposed new paragraph would also provide that any variations must be used consistently throughout the label and SDS. Because of the proposed addition of new paragraph C.2.4.7, OSHA is also proposing to renumber existing paragraphs C.2.4.7 and C.2.4.8 to become C.2.4.8 and C.2.4.9, respectively.
OSHA is also proposing to add a new paragraph, C.2.4.10, to further address cases where substances or mixtures may trigger multiple precautionary statements for medical responses. Consistent with the GHS Rev. 7 (UN GHS, 2017, Documents ID 0060), OSHA is proposing principles for addressing situations where a substance or mixture is classified for a number of hazards and triggers multiple precautionary statements for medical responses (e.g., calling a poison center/doctor/. . . . and getting medical advice/attention). Proposed paragraph C.2.4.10 would provide for a system of prioritization for precautionary statements. Under proposed C.2.4.10(a), labels would usually need only include one precautionary statement reflecting the response at the highest level with the greatest urgency, combined with at least one route of exposure or symptom "IF" statement. For example, the statement, "Immediately call a poison center/doctor/. . ." would be prioritized over the less urgent "call a poison center/doctor."
OSHA believes there is value in including more than one precautionary statement related to medical response to address both immediate (acute) and long-term (chronic) medical concerns; appropriate medical care may be different depending on whether there is a medical emergency (e.g., chemical burns) or concerns about potential diseases (e.g., cancer) due to prolonged exposures. However, OSHA also understands the difficulty involved in providing a long list of medical responses and that this could lead to confusion, particularly when immediate action is required. Therefore, proposed paragraph C.2.4.10(b) would allow for (but not require) combination of medical response statements. This means that if a chemical has, for example, inhalation and skin contact hazards that would require the same level of medical response, both of these routes of entry could be listed in a combined statement. Proposed paragraph C.2.4.10(c) would prohibit the combination of medical response statements where the statements "Get medical advice/attention if you feel unwell" and "Get immediate medical/advice attention" are both indicated. In those cases, both statements should appear without prioritization. OSHA is requesting comments on whether precautionary statements for medical responses should be prioritized and seeks input on the best method(s) to use for prioritization.
Proposed Revisions to C.4
OSHA is proposing to update the hazard label elements for specific hazard classes and categories. The following discussion on proposed revisions to C.4 is organized according to: (1) Labeling changes resulting from the addition of hazard classes and categories in appendix B (new subcategories for flammable gases (C.4.15), Aerosols category 3 (C.4.16), and desensitized explosives (C.4.30)); (2) revisions to hazard statements, hazard categories and notes; (3) revisions to precautionary statements; and (4) the GHS revisions that OSHA is not proposing to adopt. In the discussion of precautionary statements, OSHA will explain the proposed changes to the statements and indicate what hazard classes/categories trigger these statements. As noted previously, a redline strike out version of appendix C is available in the docket and on OSHA's website so interested parties can see all of the proposed changes in context (OSHA HCS Redline, 2020, Document ID 0222; https://www.osha.gov/dsg/hazcom/).
Proposed Revisions Based on Additions of Hazard Classes and Categories
OSHA is proposing a number of consequential revisions to appendix C based on the proposed additions of hazard classes and categories to appendix B. As discussed in the Summary and Explanation for appendix B, OSHA is proposing a number of changes to the flammable gas hazard class. The changes would include: (1) Subdividing category 1 flammable gases into categories 1A and 1B; (2) adding pyrophoric gases into category 1A; and (3) adding chemically unstable gases into category 1A (further subdivided into chemically unstable gas A and chemically unstable gas B). The proposed hazard and precautionary statements for those gases, consistent with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060) are in C.4.15. Each type of category 1A gas (including pyrophoric gases and chemically unstable gases) would require the hazard statement "Extremely flammable gas," as is currently required for category 1 gases. On the other hand, the hazard statement for the new category 1B flammable gases would be "Flammable gas." Additional hazard and precautionary statements would be added to communicate hazards specific to, and precautions that need to be taken for, pyrophoric and chemically unstable gases.
As also discussed in the Summary and Explanation for appendix B, OSHA is proposing to add non-flammable aerosols to the existing "Flammable Aerosols" hazard class and to rename the class "Aerosols." Consequently, in appendix C, OSHA proposes to adopt the GHS Rev. 7 (UN GHS, 2017, Document ID 0060) hazard and precautionary statements for non-flammable aerosols in C.4.16. OSHA believes that these communication requirements would better address the true hazards of aerosols. In cases where aerosols are currently labeled as gases under pressure, the proposal would require the label to be updated to include the flame pictogram for hazard categories 1 and 2 (no pictogram would be required for hazard category 3) and the signal word "warning" (if "danger" is not required due to flammability); the hazard statement "pressurized container, may burst if heated" would also be required. These changes would better differentiate the hazards of non-flammable aerosols from those of gases under pressure.
Finally, OSHA is also proposing to adopt the hazard class of desensitized explosives in appendix B, and consequently to adopt, in appendix C, the pictogram, signal word, hazard statements, and precautionary statements for desensitized explosives from the GHS Rev. 7 (UN GHS, 2017, Document ID 0060). Under the proposal, the labeling information for desensitized explosives would be at C.4.30.
For flammable gases, aerosols, and desensitized explosives, OSHA is proposing to adopt the GHS Rev. 7 (UN GHS, 2017, Document ID 0060) hazard communication information with only minor editorial revisions, such as the use of HCS instead of GHS terminology (e.g., "manufacturer, importer or distributor" instead of "manufacturer/supplier or the competent authority" in conditional instructions). OSHA believes that the information called for by the GHS Rev. 7 (UN GHS, 2017, Document ID 0060) effectively communicates the hazards of those substances and the precautions that need to be taken when handling them. Start Printed Page 9714 Therefore, requiring the information to appear on labels would improve hazard communication and enhance worker safety. In addition, because the changes would align the HCS with the GHS, adopting them would ease compliance burdens for U.S. stakeholders that must also comply with international requirements for hazard communication.
Proposed Revisions to Hazard and Precautionary Statements, Hazard Categories, and Notes
OSHA is proposing to revise a number of hazard and precautionary statements to align with the GHS Rev. 7. The hazard and precautionary statements in the current HCS were adopted from the GHS Rev. 3. Since the HCS was last updated in 2012, the UNSCEGHS has continued to discuss the utility and readability of the label elements, including hazard and precautionary statements, in order to improve the information presented. The specific goals of the UNSCEGHS are to make labeling information more comprehensible and useable by explaining and clarifying ambiguous or unhelpful instructions or statements and eliminating inconsistencies in statements (UN GHS, 2018, Document ID 0095; UN GHS, 2018, Document ID 0213). In addition, the UNSCEGHS is considering how precautionary statements could be consolidated or combined to save label space and make labels more readable and clear, all of which improve the safety message (UN GHS, 2018, Document ID 0095; UN GHS, 2018, Document ID 0213). OSHA shares these goals with the UNSCEGHS because they lead to better communication of hazards and therefore maintain or enhance protection of worker safety and health. Unless otherwise discussed below, OSHA is proposing to adopt the updated communication information presented in the GHS Rev. 7 (UN GHS, 2017, Document ID 0060) with only minor editorial revisions, such as using the HCS terminology instead of the GHS terminology (e.g., "manufacturer, importer or distributor" instead of "manufacturer/supplier or the competent authority" in conditional instructions).
Proposed Revisions to Tables C.4.1, C.4.2, and C.4.3 (Acute Toxicity Tables)
OSHA is proposing to consolidate hazard category information for acute toxicity—oral, C.4.1. The change would involve deleting the table for acute toxicity—oral, category 3 and combining categories 1, 2, and 3 in one table, since all three categories have the same precautionary statements. None of the substantive communication information for categories 1, 2, or 3 would change, and the intent of the proposed modification is simply to make C.4.1 more concise.
Proposed Revisions to Precautionary Statements
The original GHS (UN GHS Rev. 1, 2005, Document ID 0215) precautionary statements were developed from existing classification systems, including the IPCS International Chemical Safety Card (ICSC) Compilers Guide (IPCS International, 2012, Document ID 0158), the American National Standards (ANSI Z129.1 2010, Document ID 0102), the EU classification and labelling directives, the Emergency Response Guidebook (UN TDG, 2016, Document ID 0218), and the Pesticide Label Review Manual of the United States Environmental Protection Agency (EPA, 2018, Document ID 0056). Since publication of the updates to the HCS in 2012, the UNSCEGHS has continued its ongoing review of the precautionary statements to ensure they are allocated to the correct hazard class and/or category, reduce redundancies, simplify and clarify the statements, and clarify and refine the conditions of use. This section discusses OSHA's proposed revisions to precautionary statements in appendix C.4. The intent or reasons provided below for the proposed changes reflect OSHA's preliminary agreement with explanations provided by the UNSCEGHS, unless otherwise specified. The changes are organized according to the column headings found in the C.4 tables (i.e., prevention, response, storage, and disposal).
Proposed Changes in Prevention Column
Wear protective equipment (e.g., gloves/protective clothing).
A precautionary statement for acute toxicity—dermal (categories 1-4) (Table C.4.2), skin corrosion/irritation (categories 1A-1C) (Table C.4.4), eye damage/irritation (categories 1 and 2A) (Table C.4.5), and sensitization—skin (Table C.4.7) specifies personal protective equipment, such as "wear protective gloves" or "wear eye protection/face protection." Instructions for the statement currently indicate that the chemical manufacturer, importer, or distributer is "to specify type of equipment." OSHA proposes to revise the instruction to state that the chemical manufacturer, importer, or distributor may further specify type of equipment where appropriate. The intent of this proposed revision is to clarify that label preparers may provide additional specification about the type of protective equipment, where appropriate, and to align with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060).
Because specific hazards may require specific protective equipment or instructions, current precautionary statements and instructions for certain health hazards (e.g., germ cell mutagenicity, see Table C.4.8; carcinogenicity, see Table C.4.9; and reproductive toxicity, see Table C.4.10) and the majority of physical hazard classes specify one or more types of personal protective equipment and indicate that the chemical manufacturer, importer, or distributor is to specify the type of equipment. The types of equipment currently listed in the HCS were adopted from the GHS Rev. 3 and vary for the different hazard classes. In 2010, the UNSCEGHS recommended that the precautionary statement "Wear protective gloves/protective clothing/eye protection/face protection" be used for the hazard classes of germ cell mutagenicity (C.4.8), carcinogenicity (C.4.9), reproductive toxicity (C.4.10), explosives (C.4.14) and unstable explosives (C.4.30) (UN GHS, 2010, Document ID 0149), and this statement was included in the HCS in 2012. In 2015, the UNSCEGHS noted that hearing protection should often be worn when handling explosives and other physical hazards, such as desensitized explosives, because an explosion would result in a potentially hazardous noise level (UN GHS, 2015, Document ID 0219). Accordingly, the UNSCEGHS revised the precautionary statement to read, "Wear protective gloves/protective clothing/eye protection/face protection/hearing protection. . ." (UN GHS, 2016, Document ID 0147). Adding the term "/hearing protection. . ." provides flexibility because hearing protection and other equipment can be selected when appropriate and not selected if not relevant. Adding the ellipsis at the end of the statement allows other types of personal protective equipment to be listed as necessary. The UNSCEGHS also revised the instruction for the precautionary statement to make it clear that it is referring to personal protective equipment. Consistent with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060) OSHA is proposing to adopt this revised precautionary statement and instruction for all relevant hazards: germ cell mutagenicity (C.4.8), categories 1A, 1B, and 2; carcinogenicity (C.4.9), categories 1A, 1B, and 2; reproductive toxicity (C.4.10), categories 1A, 1B, and 2; explosives (C.4.14), Start Printed Page 9715 unstable and division 1.1-1.5; flammable gases (C.4.15), category 1A, pyrophoric; flammable liquids (C.4.19), categories 1, 2, 3, and 4; flammable solids (C.4.20), categories 1 and 2; self-reactive substances and mixtures (C.4.21), categories Types A, B, C, D, E, and F; pyrophoric liquids (C.4.22), category 1; pyrophoric solids (C.4.23), category 1; self-heating substances and mixtures (C.4.24), categories 1, and 2; substances and mixtures which, in contact with water, emit flammable gases (C.4.25), categories 1, 2, and 3; oxidizing liquids (C.4.26), categories 1, 2, and 3; oxidizing solids (C.4.27), categories 1, 2, and 3; organic peroxides (C.4.28), categories Types A, B, C, D, E, and F; and desensitized explosives (proposed new C.4.30), categories 1, 2, 3, and 4.
Avoid Contact During Pregnancy/While Nursing
In Table C.4.10, for reproductive toxicity (effects on or via lactation), OSHA is proposing to revise a precautionary statement that currently says to avoid contact "during pregnancy/while nursing" so it reads "during pregnancy and while nursing." This proposed revision would clarify that the chemical manufacturer, importer or distributor is not to choose between "during pregnancy" and "while nursing" but is to include both scenarios on the label. This proposed change would align with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2012, Document ID 0155).
Do Not Handle Until all Safety Precautions Have Been Read and Understood
For unstable explosives (Table C.4.14), OSHA is proposing to delete the precautionary statement about not handling until all safety precautions have been read and understood. A statement to obtain special instructions before use is already included and that statement is shorter and more relevant to safety. This proposed change would align with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2012, Document ID 0155).
Do Not Subject to Grinding/Shock/Friction
OSHA also proposes adding the precautionary statement "Do not subject to grinding/shock/friction/. . ." to the table for unstable explosives (Table C.4.14). That statement is already included for the other explosives categories, and is also relevant for unstable explosives. For each of the explosives categories that contain that statement, an explanatory conditional note clarifying that the statement applies only if the explosive is mechanically sensitive would also be added. These proposed changes would align with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2012, Document ID 0152; UN GHS 2012, Document ID 0153).
Keep Away From Heat/Sparks/Open Flames/Hot Surfaces
A number of the hazard classes that include flammable chemicals currently require precautionary statements and instructions about keeping away from ignition sources (heat/sparks/open flames/hot surfaces). Those statements generally require the chemical manufacturer, importer, or distributor to select one or more of the ignition sources listed, as applicable. OSHA is proposing to include more ignition sources in the statement and to require that they all be listed on the label. With that change, the statement would read, "Keep away from heat, hot surfaces, sparks, open flames, and other ignition sources." OSHA believes this change, which is consistent with the GHS Rev.7 (UN GHS, 2017, Document ID 0060; UN GHS, 2012, Document ID 0152), would improve hazard communication by making users aware of additional ignition sources that should be avoided. The change would be made to precautionary statements for explosives (divisions 1.1-1.5 in Table C.4.14), flammable gases (Table C.4.15), aerosols (Table C.4.16), flammable liquids (Table C.4.19), flammable solids (Table C.4.20), self-reactive substances and mixtures (Table C.4.21), pyrophoric liquids (Table C.4.22), pyrophoric solids (Table C.4.23), oxidizing liquids (Table C.4.26), oxidizing solids (Table C.4.27), organic peroxides (Table C.4.28), and desensitized explosives (Table C.4.30).
Keep Wetted With
A conditional instruction used for division 1.1-1.3 and 1.5 explosives in Table C.4.14 currently states that the chemical manufacturer, importer, or distributer is to include the precautionary statement "Keep wetted with. . ." under conditions where drying would increase the explosion hazard, except as needed for manufacturing or operating processes. The GHS Rev. 7 changes the conditional instruction to clarify that the "Keep wetted with. . ." statement should be used for "substances or mixtures which are wetted, diluted, dissolved or suspended with a phlegmatizer to reduce or suppress their explosive properties" (UN GHS, 2017, Document ID 0060; UN GHS, 2012, Document ID 0152; UN GHS, 2012, Document ID 0153). OSHA is proposing to make the same change in order to clarify when the "Keep wetted with. . ." statement is appropriate.
The "Keep wetted with. . ." precautionary statement also appears in proposed C.4.30, desensitized explosives. Consistent with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060), OSHA is not proposing to add the conditional statement that appears in C.4.14 because, by definition, desensitized explosives are phlegmatized to suppress their explosive properties, and therefore the "Keep wetted with. . ." statement is appropriate for all desensitized explosives. OSHA requests comment on these proposed changes.
Keep Only in Original Container
OSHA proposes to revise the statement "Keep only in original container" to "Keep only in original packaging" for self-reactive substances and mixtures (Table C.4.21), organic peroxides (Table C.4.28), and corrosive to metals (Table C.4.29). The revised statement would also be added to explosives in division 1.1-1.5 (Table C.4.14). OSHA believes that this proposed change is appropriate because the term "packaging" is more inclusive than "container" and would include the transport packaging as well as the immediate container. These proposed changes are consistent with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2012, Document ID 0152).
Ground/Bond Container and Receiving Equipment
Several hazard classes require the precautionary statement "Ground/bond container and receiving equipment" for chemicals that are electrostatically sensitive. OSHA proposes changing "Ground/bond" to "Ground and bond" to clarify that both of those precautions are to be included on the label. Appendix C.2.4.2, states that when a "/" is used the label preparer has a choice and should choose the most appropriate phrase. However, in this case, both "ground and bond" should be stated together to appropriately protect against electrostatically sensitive chemicals. These proposed changes would apply to explosives (division 1.1-1.5 in Table C.4.14), flammable liquids (categories 1-3 in Table C.4.19), and flammable solids (Table C.4.20). In addition, OSHA is proposing to revise existing conditional instructions to clarify that the need for grounding and bonding applies to flammable liquids only if they are volatile and may generate an explosive atmosphere (Table C.4.19) and to Start Printed Page 9716 explosives and flammable solids only if they are electrostatically sensitive (Tables C.4.14 and C.4.20). OSHA is also proposing to add the "ground and bond" precautionary statement and similar conditional notes ("if electrostatically sensitive and able to generate an explosive atmosphere") to self-reactive substances and mixtures (Table C.4.21) and organic peroxides (Table C.4.28) because the precaution is also appropriate for those hazard classes. These proposed changes would align with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2012, Document ID 0152).
Keep/Store Away From Clothing, and Other Combustible Materials
OSHA is proposing to standardize precautionary statements regarding combustible materials for oxidizing chemicals. Currently, the tables for oxidizing gases (Table C.4.17), oxidizing liquids (Table C.4.26, hazard categories 2 and 3), and oxidizing solids (Table C.4.27, hazard categories 2 and 3) require the precautionary statement "Keep/Store away from clothing/. . . /combustible materials," along with instructions for the chemical manufacturer, importer, or distributor to specify incompatible materials. OSHA proposes to change the statement to read: "Keep away from clothing and other combustible materials," and to delete the instruction regarding incompatible materials, to make the statement more consistent with the statement currently applicable to hazard category 1 in both oxidizing liquids (Table C.4.26) and oxidizing solids (Table C.4.27). OSHA believes the proposed change is appropriate because the general term "combustible materials" encompasses any other materials that are incompatible with oxidizers. In addition, OSHA believes the term "keep" is adequate to encompass storage as well as use, and that eliminating the choice between "keep" and "store" would avoid confusion and improve consistency. Finally, OSHA is also proposing to remove the redundant statement "Take any precaution to avoid mixing with combustibles/. . ." under oxidizing liquids (Table C.4.26) and oxidizing solids (Table C.4.27), since this information is duplicative of the "keep away from" statement. These proposed changes are consistent with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2012, Document ID 0152).
OSHA is proposing to remove the "keep/store away from clothing/. . . /combustible materials" precautionary statement, along with its instruction, for self-reactive substances and mixtures (Table C.4.21) and organic peroxides (Table C.4.28). The wording of the precautionary statement is pertinent to oxidizing properties, which readily give oxygen or other oxidizing material, and therefore more readily support combustion. Neither self-reacting chemicals nor organic peroxides have oxidizing properties, so the statement is not appropriate for them. Both self-reacting chemicals and organic peroxides have alternate storage statements that are designed to more accurately address their particular chemical properties. These proposed changes would also align with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2012, Document ID 0152; UN GHS, 2012, Document ID 0153).
Keep Valves and Fittings Free From Oil and Grease
For oxidizing gases (Table C.4.17), a precautionary statement currently allows the chemical manufacturer, importer, or distributor to specify that either "reduction valves" or "valves and fittings" be kept free from oil and grease. OSHA is proposing to revise the statement to "Keep valves and fittings free from oil and grease." OSHA believes the change is appropriate because all valves and fittings must be kept free of oil and grease, not just the reduction valves attached to pressure receptacles. This proposed change is consistent with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2010, Document ID 0149).
Wear Cold Insulating Gloves/Face Shield/Eye Protection
OSHA is proposing to revise the precautionary statement for refrigerated liquefied gases (Table C.4.18), which currently provides that either cold insulated gloves, a face shield, or eye protection is to be used. The proposed change would clarify the intent of the precautionary statement, which is that cold-insulating gloves are to be used in addition to either a face shield or eye protection. This proposed change would align with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2012, Document ID 0152; UN GHS, 2012, Document ID 0153).
Keep Container Tightly Closed
The precautionary statement "Keep container tightly closed" is used for flammable liquids (categories 1-3 in Table C.4.19). The GHS Rev. 7 contains a conditional instruction indicating that the statement is to be used if the liquid is volatile and may generate an explosive atmosphere (UN GHS, 2017, Document ID 0060). OSHA is proposing to add this conditional instruction to the precautionary statement for flammable liquids (categories 1-3) because it clarifies the types of flammable liquids for which the statement applies.
OSHA also proposes to add the precautionary statement "Keep container tightly closed" to pyrophoric liquids (Table C.4.22) and pyrophoric solids (Table C.4.23). OSHA believes it is important to add that statement because for both pyrophoric liquids and pyrophoric solids it is necessary to avoid ignition via contact with air. Because the precaution applies to all chemicals in these hazard classes, OSHA does not believe a conditional note is necessary. These proposed changes would also align with the GHS, Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2012, Document ID 0152; UN GHS, 2012, Document ID 0153).
Take Precautionary Measures Against Static Discharge
For flammable liquids (Table C.4.19, hazard categories 1-3), OSHA proposes to revise the precautionary statement "Take precautionary measures against static discharge" to "Take action to prevent static discharge." The revision would simply shorten the statement and clarify what action needs to be taken. OSHA also proposes to add a note that this precautionary statement is to be used if the liquid is volatile and may generate an explosive atmosphere. These proposed changes are consistent with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2012, Document ID 0152; UN GHS, 2012, Document ID 0153).
Flammable Liquids/Solids Conditional Instructions
OSHA is proposing additional conditional instructions for flammable liquids (Table C.4.19) and flammable solids (Table C.4.20). Some categories of flammable liquids (categories 1-3) and flammable solids (categories 1 and 2) contain a precautionary statement specifying the use of "explosion-proof [electrical/ventilating/lighting/. . .] equipment." OSHA believes that SDS and label creators are not actually properly and specifically identifying the prevention measures for the particular chemical, but rather are listing the entire line without the required details. For liquids, OSHA proposes a new conditional instruction to clarify that the statement is required if the chemical is volatile and may generate an explosive atmosphere. For both liquids and solids, a conditional instruction would be added to indicate that text in Start Printed Page 9717 square brackets may be used to specify specific electrical, ventilating, lighting or other equipment if necessary and as appropriate. These proposed changes would align with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2012, Document ID 0152; UN GHS, 2012, Document ID 0153).
OSHA is also proposing to add a conditional instruction to the precautionary statement to use non-sparking tools for flammable liquids (categories 1-3, Table C.4.19). The statement would clarify that the precautionary statement is only needed if the liquid is volatile and may generate an explosive atmosphere, and if the minimum ignition energy is very low (<0.1 mJ). The precautionary statement has very limited applicability for flammable liquids and therefore OSHA believes that the conditions need to be specified. This proposed change is also consistent with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2012, Document ID 0152; UN GHS, 2012, Document ID 0153).
Keep Cool
For self-reactive substances and mixtures and organic peroxides (Tables C.4.21 and C.4.28), OSHA is proposing to move the precautionary statement "Keep cool" from the storage column to the prevention column. The precautionary statement is not needed in the storage column because that column includes a precautionary statement about storage temperatures not to be exceeded, and as discussed below, OSHA is proposing to add conditional instructions to that column to inform users of when a storage temperature would need to be listed. Under the prevention column, OSHA is proposing to include a conditional instruction indicating that the precautionary statement may be omitted if storage temperatures are included on the label. This proposed revision would not materially change the information that is presented on the label, and is consistent with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2012, Document ID 0152; UN GHS, 2012, Document ID 0153).
For self-heating substances and mixtures (Table C.4.24), a combined precautionary statement currently instructs the user to keep cool and protect from sunlight. OSHA is proposing that a conditional instruction be added to indicate that "Keep cool" can be omitted where storage temperatures are listed on the label. Because "Protect from sunlight" still needs to be included if specific storage temperatures are listed on the label, OSHA is proposing to delete the combined statement under the prevention column, and to list only "Keep cool" (and the new conditional instruction) in that column. The statement: "Protect from sunlight" would be moved to the storage column, similar to the way this is handled for other hazard classes. OSHA believes that these proposed changes would provide the label preparer better instructions and would provide the appropriate level of information on the label without repetition. These proposed changes would also align with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2012, Document ID 0152; UN GHS, 2012, Document ID 0153).
Do Not Allow Contact With
OSHA is proposing to add the conditional note "if emphasis of the hazard statement is deemed necessary" to precautionary statements indicating that contact is not to be allowed with air (for pyrophoric gases (proposed C.4.15, category 1A), pyrophoric liquids (C.4.22), and pyrophoric solids (C.4.23)) or water (for substances and mixtures that emit flammable gases in contact with water (C.4.25, categories 1 and 2). Because the hazard phrases, which are also included on labels for these categories, already warn about the hazards of these respective chemicals when they contact air or water, adding this precautionary statement as well could be repetitive. However, depending on the specific chemical, the label preparer may feel that added emphasis is warranted. These proposed changes would align with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2012, Document ID 0152; UN GHS, 2012, Document ID 0153).
Handle Contents Under Inert Gas
For substances and mixtures which, in contact with water, emit flammable gases (Table C.4.25), OSHA proposes changing the precautionary statement "Handle under inert gas. Protect from moisture" to "Handle and store contents under inert gas/. . . Protect from moisture." This would clarify that these substances should always be under inert atmospheres. In addition, conditional instructions would be added to indicate that if the substance or mixture reacts readily with moisture in air, then the chemical manufacturer, importer or distributer also has to specify the appropriate liquid or gas if inert gas is not appropriate. The new statement would provide greater clarity and is needed because inert gas is not appropriate in some cases (e.g., white phosphorus should be handled and stored under water) (UN GHS, 2010, Document ID 0149). This proposed change is consistent with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2012, Document ID 0152; UN GHS, 2012, Document ID 0153).
OSHA is also proposing to add the statement "Handle and store contents under inert gas/. . ." to pyrophoric liquids (C.4.22) and pyrophoric solids (C.4.23). A conditional statement would note that the manufacturer, importer, or distributor is to specify the appropriate liquid or gas if inert gas is not appropriate. Pyrophoric chemicals, by definition, are likely to ignite when in contact with air. Both Tables C.4.22 and C.4.23 currently contain the following statement in the storage column: "Store contents under . . . Chemical manufacturer, importer, or distributor to specify appropriate liquid or inert gas." In light of the language OSHA is proposing to include in the prevention column, OSHA would delete this language from the storage column. OSHA believes that the language being proposed for the prevention column would emphasize that pyrophoric chemicals must be handled, as well as stored, under inert atmospheres. OSHA notes that the statements OSHA is proposing to add to the prevention column for Tables C.4.22 (pyrophoric liquids) and C.4.23 (pyrophoric solids) regarding handling and storing contents under inert gas were included in the GHS Rev. 5, but were inadvertently omitted from Rev. 7 (UN GHS, 2016, Document ID 0211; UN GHS, 2017, Document ID 0060). If OSHA finalizes the language as proposed, it will work with the UNSCEGHS to have this statement reinstated in future GHS revisions.
Wear Fire/Flame Resistant/Retardant Clothing
Category 1 oxidizing liquids (C.4.26) and category 1 oxidizing solids (C.4.27) currently have the precautionary statement "Wear fire/flame resistant/retardant clothing." The intent of that statement is to alert the users of the chemical that they should wear either fire resistant or flame retardant clothing, not for the label preparer to choose between the terms "fire" and "flame" or "resistant" and "retardant". Therefore, OSHA proposes to replace the existing statement with "Wear fire resistant or flame retardant clothing." This would clarify the intent of this statement and is consistent with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2012, Document ID 0152; UN GHS, 2012, Document ID 0153). Start Printed Page 9718
Proposed Changes in Response Column
For the response column, a number of the proposed revisions in appendix C are simply editorial and are made to improve clarity, correct simple omissions of a word or phrase, or more efficiently and concisely combine different precautionary statements. For example, OSHA is proposing to add the phrase "If on skin" to the statement "Brush off loose particles from skin" (see C.4.23 (pyrophoric solids) and hazard categories 1 and 2 in C.4.25 (substances and mixtures which, in contact with water, emit flammable gasses)) because those statements are always combined in the GHS Rev. 7 (UN GHS, 2017, Document ID 0060), and the additional phrase would add clarity. Another example is OSHA's proposal to add the phrase "In case of fire" at the beginning of the precautionary statements related to fire fighting for unstable explosives, as is already done for other explosives categories (see C.4.14). In a number of cases, OSHA is proposing to reorganize the precautionary statements and to remove redundant wording to improve clarity. For example, in C.4.14, instead of listing the individual statements and providing conditions of use, OSHA would now list the statements grouped together (except for materials for Division 1.4S, which have another set of statements as explained below).
The following discussion does not address proposed changes that are simply editorial in nature (although all proposed revisions can be found in the redlined version of appendix C that is available as part of the rulemaking record (OSHA HCS Redline, 2020, Document ID 0222) and on OSHA's website (https://www.osha.gov/dsg/hazcom). The discussion below highlights the substantive changes OSHA is proposing to make to the response column in appendix C.
Take Off Immediately All Contaminated Clothing. Rinse Skin With Water/Shower
The existing precautionary statements for skin corrosion/irritation (categories 1A to 1C in C.4.4) and flammable liquids (categories 1-3 in C.4.19) indicate that if the chemical is on hair or skin, the affected individual is to immediately take off all contaminated clothing and rinse skin with "water/shower." OSHA proposes to revise the statement to instruct the affected individual to rinse skin with "water [or shower]," and to add a conditional note indicating that the text in square brackets is to be used where the chemical manufacturer, importer or distributor considers it appropriate for the specific chemical. The reason for the proposed change is that a deluge shower might be most appropriate for the chemical, and the use of the square brackets allows for selection of the most appropriate wording. The proposed change would align with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2012, Document ID 0152; UN GHS, 2012, Document ID 0153).
Get Medical Advice/Attention
A number of health hazards (i.e., skin corrosion/irritation (category 2 in Table C.4.4), eye damage/irritation (categories 2A and 2B in Table C.4.5), sensitization—skin (Table C.4.7), germ cell mutagenicity (Table C.4.8), carcinogenicity (Table C.4.9), reproductive toxicity (Table C.4.10), specific target organ toxicity—repeated exposure (Table C.4.12), and refrigerated liquefied gases (Table C.4.18)) have combined precautionary statements that include the statement "get medical advice/attention." OSHA is proposing to add an instruction indicating that the chemical manufacturer, importer, or distributer is to select medical advice or attention as appropriate. This is to alert label preparers that they should provide more specific instruction on the type of medical assistance needed based on the chemical hazard and to align with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060).
If Breathing Is Difficult, Remove Person to Fresh Air and Keep Comfortable for Breathing
A precautionary statement used for sensitization—respiratory (Table C.4.6) currently states "If inhaled: If breathing is difficult, remove person to fresh air and keep comfortable for breathing." OSHA is proposing to remove the phrase "if breathing is difficult." This is because including two conditions, "if inhaled" and "if breathing is difficult," is confusing and unnecessary. Removal of the phrase would also make the precautionary statement consistent with the statement as it appears in other hazard classes in appendix C.4, such as acute toxicity—inhalation (Table C.4.3). This proposed change is consistent with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2010, Document ID 0149).
Take Off Contaminated Clothing and Wash it Before Reuse
A precautionary statement for skin sensitization (Table C.4.7) currently says to wash contaminated clothing before reuse. OSHA is proposing to add the phrase "Take off contaminated clothing and" to this precautionary statement. The UNSCEGHS previously recommended that this additional phrase be used for acute toxicity—dermal; skin irritation, category 2; and sensitization—skin (UN GHS, 2010, Document ID 0154). The phrase was inadvertently omitted for skin sensitization in the GHS Rev. 3 (UN GHS, 2009, Document ID 0085), and accordingly in the updates to the HCS in 2012, but it has since been added to the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2010, Document ID 0149; UN GHS, 2010, Document ID 0154).
If Exposed or Concerned
For specific target organ toxicity (single exposure) (Table C.4.11), OSHA is proposing to revise a precautionary statement indicating "If exposed" to "If exposed or concerned." The revision, which would be consistent with language currently used for the germ cell mutagenicity (Table C.4.8), carcinogenicity (Table C.4.9), and reproductive toxicity (Table C.4.10) hazard classes, would maintain consistency throughout C.4 and with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060). In 2010, a GHS subcommittee recommended that wherever "If exposed" is used, it be revised to "If exposed or concerned," since the user of the chemical may not have evidence of exposure (UN GHS, 2010, Document ID 0154).
Division 1.4 Explosives (C.4.14) Precautionary Statements
For Division 1.4 explosives, the HCS currently provides fire-fighting precautionary statements and instructions on when to apply them (Table C.4.14). OSHA is proposing two changes to these statements. First, OSHA is proposing to change the instruction note from "for explosives are 1.4S ammunition and components thereof" to "for explosives of division 1.4 (compatibility group S) in transport packaging." This revision would provide clarity about when the note applies and there is no intended change in meaning. Second, OSHA is proposing to revise the precautionary statement "Fight fire with normal precautions from a reasonable distance" to the statement "Fight fire remotely due to the risk of explosion." OSHA believes the proposed new statement is more appropriate and protective because it specifies the explosion risk due to fire associated with 1.4 compatibility group S (1.4S) explosives. These proposed changes would align with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2012, Document ID 0152; UN GHS, 2012, Document ID 0153). Start Printed Page 9719
Eliminate All Ignition Sources, if Safe To Do So
For category 1 and 2 flammable gases (C.4.15), a precautionary statement currently instructs the user to eliminate all ignition sources if safe to do so. OSHA proposes to revise the statement to "In case of leakage, eliminate all ignition sources." The term "in case of leakage" would be added to stress that it is important to eliminate flammable gas leaks, even where the leaking gas is not burning, because the leak could create an explosive atmosphere. The term "if safe to do so" would be deleted because it could discourage quick action. Eliminating gas leaks would not be expected where a fire would hinder that action. OSHA is also proposing to add this statement to pyrophoric gases 1A and chemically unstable gases A and B. These proposed changes would be consistent with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UNGHS, 2012, Document ID 0152).
In Case of Fire Use . . . To Extinguish
For self-reactive substances and mixtures (type A) (C.4.21), OSHA is proposing to delete the precautionary statements "In case of fire use . . . to extinguish" (along with its explanatory note) and "Fight fire remotely due to the risk of explosion." In place of the language OSHA is proposing to delete, OSHA proposes to use language stating "In case of fire: Explosion risk. Do NOT fight fire when fire reaches explosives." These changes would align with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060) and are proposed because it is dangerous to fight a fire involving this type of material and individuals should always be advised against it (UN GHS, 2012, Document ID 0152; UN GHS, 2012, Document ID 0153). OSHA is not proposing to change the existing statement about evacuating the area.
For type B self-reactive substances and mixtures (C.4.21), OSHA is proposing to combine existing precautionary statements and to delete duplicate phrases that would occur with the new combination. OSHA does not intend these changes to alter the meaning of the statements. OSHA is proposing to use brackets around the statement "Use . . . to extinguish" with a conditional note to indicate that the text in square brackets is to be included if water increases risk. This is to preserve the conditions of use with the new combination of phrases. These proposed changes would align with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2012, Document ID 0095).
Fire and Explosion Hazards for Organic Peroxides (C.4.28)
Precautionary statements and instructions related to fire and explosion hazards or fire-fighting procedures were not included in the GHS Rev. 3 (UN GHS, 2009, Document ID 0085), or in the current HCS, for organic peroxides (C.4.28). The UNSCEGHS has since adopted these precautionary statements (UN GHS, 2012, Document ID 0095). As in GHS Rev. 7 (UN GHS, 2017, Document ID 0060), OSHA is proposing to adopt the same precautionary statements in the response column for organic peroxides (C.4.28) as for self-reactive substances and mixtures (C.4.21). OSHA believes it is appropriate to include these statements for organic peroxides, as well as for self-reactive substances and mixtures, because the fire and explosion hazards of the two classes of compounds are equivalent (UN GHS, 2012, Document ID 0152; UN GHS, Document ID 0153; UN GHS, 2012, Document ID 0095).
Immerse in Cool Water/Wrap With Wet Bandages
For pyrophoric liquids (C.4.22), pyrophoric solids (C.4.23), and substances and mixtures which in contact with water emit flammable gases (C.4.25), a precautionary statement currently indicates that if the substance is on the skin, the user should "immerse in cool water/wrap with wet bandages." For pyrophoric liquids (C.4.22) and solids (C.4.23), OSHA is proposing to change the forward slash to an "or" so that the statement would read "Immerse in cool water or wrap in wet bandages." The change is proposed to make clear that the chemical manufacturer, importer, or distributer is not to choose one action or the other but is to include both actions on the label. In the case of substances and mixtures which, in contact with water, emit flammable gases, OSHA is proposing to delete "wrap in wet bandages" from the statement so that the complete statement reads "Brush off loose particles from skin and immerse in cool water." This change is proposed because, for these chemicals, a large volume of water is needed and wrapping in wet bandages is not enough to address problems caused by the heat of the reaction (UN GHS, 2012, Document ID 0095). These proposed changes would align with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2012, Document ID 0152; UN GHS, 2012, Document ID 0153).
Proposed Changes in Storage Column
Store Away From Other Materials
For self-reactive substances and mixtures (C.4.21), self-heating substances and mixtures (C.4.24), and organic peroxides (C.4.28), OSHA proposes to revise the precautionary statement "Store away from other materials" to "Store separately." OSHA believes that the revised statement is preferable because it is shorter and more appropriate. OSHA is also proposing to add the "Store separately" precautionary statement to category 1 oxidizing liquids (C.4.26) and category 1 oxidizing solids (C.4.27) because those chemicals are not compatible with other chemicals and thus must be stored separately. These proposed changes are consistent with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2012, Document ID 0152; UN GHS, 2012, Document ID 0153).
Store Contents Under. . . .
For pyrophoric liquids (C.4.22) and solids (C.4.23), OSHA proposes to delete a precautionary statement that says "Store contents under . . . ," along with the instructional note that the chemical manufacturer, importer, or distributer is to specify the appropriate liquid or inert gas. The UNSCEGHS recommended that the statement be deleted from the storage column because it adopted the statement "Handle and store contents under inert gas/ . . . ," along with a similar instructional note, in the prevention column (UN GHS, 2012, Document ID 0152; UN GHS, 2012, Document ID 0153). OSHA believes placing the statement in the prevention column is more appropriate, as there it would warn the downstream user that pyrophoric chemicals must be under inert gas not only during storage but at all times, including during processing and use. This modification was inadvertently omitted from the text in the GHS Rev. 7, and the U.S. will work with the U.N. to submit a paper to add this statement to pyrophoric liquids (C.4.22) and solids (C.4.23) in a future revision of the GHS.
Maintain Air Gap Between Stacks/Pallets
For self-heating substances and mixtures (C.4.24), OSHA is proposing to revise the precautionary statement that currently says "[m]aintain air gap between stacks/pallets" so it reads instead "[m]aintain air gap between stacks or pallets." The change would clarify that chemical manufacturers, importers or distributors are not to choose between "stacks" or "pallets" Start Printed Page 9720 but are to include both words on the label. This proposed change would align with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2012, Document ID 0152; UN GHS, 2012, Document ID 0153).
Store in Corrosive Resistant/ . . . Container With a Resistant Inner Liner
A precautionary statement for the corrosive to metals (C.4.29) class currently says to store in a "corrosive resistant/ . . . container with a resistant inner liner." OSHA is proposing to change the word "corrosive" to "corrosion" because it is the technically correct term. In addition, a new conditional instruction would be inserted to indicate that the precautionary statement may be omitted if the statement "Keep only in original packaging" is included on the label. This would eliminate the redundancy of including both statements. These proposed changes would align with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2012, Document ID 0152; UN GHS, 2012, Document ID 0153).
Instructional Notes
For acute toxicity—inhalation (C.4.3) (category 1-3) and specific organ toxicity (single exposure, category 3) (C.4.11), OSHA is proposing minor, non-substantive edits to the conditional instruction for precautionary statements about keeping the container tightly closed and storing in a well-ventilated place. OSHA proposes to revise the note from "if product is volatile so as to generate hazardous atmosphere" to "if the chemical is volatile and may generate a hazardous atmosphere." The intent of these edits is to improve clarity and make the instruction more consistent with a newly added instruction for flammable liquids (C.4.19). This proposed change is consistent with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2012, Document ID 0152; UN GHS, 2012, Document ID 0153).
For flammable liquids (C.4.19), OSHA is proposing to add a clarifying instruction indicating that the precautionary statement "Store in a well ventilated place. Keep cool" applies to flammable liquids in category 1 and other flammable liquids that are volatile and may generate an explosive atmosphere. However, for category 4 flammable liquids, OSHA is proposing to delete "Keep cool," because these liquids are less volatile and have a flashpoint above 60 °C and therefore are unlikely to generate a hazardous concentration of vapor during storage; OSHA believes the precautionary statement "Store in a well ventilated place" is the appropriate level of protection. These proposed changes would align with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2012, Document ID 0152; UN GHS, 2012, Document ID 0153).
For explosives (C.4.14), OSHA is proposing minor edits to precautionary statements and instructions for storing in accordance with local/regional/national/international regulations. The edits are intended to clarify that the chemical manufacturer, importer, or distributer is to specify the applicable regulations. These proposed changes are consistent with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2012, Document ID 0152; UN GHS, 2012, Document ID 0153).
Aerosols (C.4.16), self-reactive substances (C.4.21), self-heating substances and mixtures (C.4.24), and organic peroxides (C.4.28) currently include precautionary statements addressing storage temperatures not to be exceeded, with temperatures listed in degrees Celsius/Fahrenheit. The GHS has added an instruction that the chemical manufacturer should use the applicable temperature scale for the region they are supplying (UN GHS, 2017, Document ID 0060; UN GHS, 2012, Document ID 0152; UN GHS, 2012, Document ID 0153). In other OSHA standards, the primary temperature scale used is Fahrenheit. Therefore, OSHA is proposing to require only the Fahrenheit scale in the precautionary statements. However, the chemical manufacturer, importer or distributor would still be permitted to include the temperature in Celsius (as noted by parens ( )) in addition to Fahrenheit.
In addition, for self-reactive substances and mixtures (C.4.21) and organic peroxides (C.4.28), OSHA proposes to add conditional instructions to two precautionary statements. The first conditional instruction would clarify that the statement to store in a well-ventilated place should not be used for temperature-controlled substances and mixtures or organic peroxides because condensation and freezing may occur. The second would clarify that a storage temperature is only needed if temperature control is required or deemed necessary. OSHA also proposes moving the precautionary statement "Keep cool" to the prevention column, as discussed above under the section on proposed changes to the prevention column. These proposed changes would align with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2012, Document ID 0152; UN GHS, 2012, Document ID 0153).
Proposed Changes in Disposal Column
For most of the health and physical hazards addressed by appendix C, the rule currently includes a precautionary statement to dispose of contents/container in accordance with local/regional/national/international regulations (to be specified). OSHA is proposing to add an instructional note in all relevant places in the appendix indicating that the chemical manufacturer, importer or distributor is to specify whether the disposal requirements apply to the contents, the container, or both. This proposed change would align with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2012, Document ID 0152; UN GHS, 2012, Document ID 0153).
The tables for explosives (C.4.14), except for hazard category division 1.6, currently include the precautionary statement to dispose of contents/container in accordance with local/regional/national/international regulations (to be specified). However, this precautionary statement may not give users the information needed to safely dispose of explosives, particularly malfunctioning, expired, or non-used explosives where special care is needed. This is of particular concern for explosives such as fireworks, signal flares and ammunition. Ill-formulated advice on the label may lead to the disposal of such explosive waste in a way that poses a risk, e.g., to the workers that handle the waste (UN GHS, 2015, Document ID 0156). Therefore, OSHA is proposing to change the precautionary note for explosives (C.4.14) to read: "Refer to manufacturer, importer, or distributor . . . for information on disposal, recovery, or recycling." An instructional note would be added to indicate that the chemical manufacturer, importer, or distributor is to specify the appropriate source of information, in accordance with local/regional/national/international regulations as applicable. The change is proposed to address the recycling or recovery of unexploded fireworks or other unused explosive cartridges and signal flares, which can result in unsafe conditions and should only be performed by specialists. This proposed change is consistent with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060; UN GHS, 2015, Document ID 0214; UN GHS, 2015, Document ID 0213).
Proposed Revisions to Label Elements for OSHA Defined Hazards (C.4.31)
OSHA is proposing a few changes to label elements for OSHA defined hazards (currently at C.4.30 and proposed to be renumbered as C.4.31). Start Printed Page 9721 This section of appendix C addresses the labeling of hazards that are not classified under the GHS, but that the HCS specifically defines as hazards that must be communicated on the label and SDS.
First, OSHA is proposing to delete the entry for "Pyrophoric Gas." In the GHS Rev. 7, pyrophoric gases are now a category under the hazard class of flammable gases, and OSHA proposes to include them there as well.
OSHA is also proposing a change to the "Combustible Dust" hazard statement. When OSHA finalized the revisions to the HCS in 2012, the GHS did not address classification of combustible dust; however, it used combustible dust as an example of "Other hazards which do not result in classification" (UN GHS, 2009, Document ID 0085). In the GHS Rev. 5, the UN updated A.4.3.2.3 to include a statement "May form explosible dust-air mixture if dispersed" for dust explosion hazards to provide guidance on the type of statement that should be used in the case of dust explosion hazards (combustible dust) (UN GHS, 2012, Document ID 0251). Subsequently, OSHA initiated UNSCEGHS discussions regarding combustible dust hazards. The UNSCEGHS adopted an annex (Annex 11) that provides additional guidance on hazard identification, the factors that contribute to a dust explosion hazard, and the need for risk assessment, prevention, mitigation, and communication (UN GHS, 2017, Document ID 0157). OSHA is now proposing to allow either the previously required statement, "may form combustible dust concentrations in air," or the statement suggested in the GHS Annex 4, "[m]ay form explosible dust-air mixture." OSHA is proposing to add square brackets after both statements containing the following language: "if small particles are generated during further processing, handling or by other means." This bracketed language is designed to indicate that this language should be added when the material can create a combustible dust hazard during the processing or handling of the chemical. OSHA is not proposing any changes to the signal word of "warning" or any pictogram requirements. These changes are the result of working papers presented to the UNSCEGHS meetings for discussion in December of 2017 (UN GHS, 2017, Document ID 0157).
GHS Revisions That OSHA Is Not Proposing To Adopt
There are a small number of revisions in the GHS Rev. 7 that OSHA is not proposing to adopt for the HCS. In general, OSHA does not propose to adopt any statements or conditional instructions that address consumer products because the HCS does not cover communication of hazards to consumers. This section discusses specific provisions in the GHS Rev. 7 (UN GHS, 2017, Document ID 0060) that OSHA is not proposing to adopt.
A number of tables for inhalation hazards in appendix C.4 (i.e., acute toxicity—inhalation (C.4.3, categories 3 and 4), respiratory sensitization (C.4.6), skin sensitization (C.4.7), and specific target organ toxicity—single exposure (C.4.11, category 3)) contain a precautionary statement that says "Avoid breathing dust/fume/gas/mist/vapors/spray." A conditional note in the GHS Rev. 7 (UN GHS, 2017, Document ID 0060) indicates that this precautionary statement is not needed where the precautionary statement "Do not breathe dust/mist/fume/gas/vapors/spray" is included on the label. Also, for skin corrosion/irritation (C.4.4, category 2), the GHS Rev. 7 (UN GHS, 2017, Document ID 0060) contains a conditional note indicating that the statement "If skin irritation occurs: Get medical advice/attention" may be omitted if the statement "If skin irritation or rash occurs: Get medical advice or treatment" is used. OSHA is not proposing to adopt these conditional instructions because it believes that proposed appendix C.2.4.8 (currently C.2.4.7), which provides instructions for the precedence of precautionary statements, already provides the necessary flexibility.
In the GHS Rev. 7, the precautionary statements about explosion-proof equipment and taking action to prevent static discharge include a conditional instruction indicating that these precautionary statements can be omitted if national or local legislation contains provisions that are more specific (UN GHS, 2017, Document ID 0060). OSHA is not proposing to adopt this instruction because the agency believes these precautionary statements contain important information that should always be included on labels. Although some OSHA and consensus standards address the use of explosion-proof equipment and preventing static discharge for flammable liquids or solids, they do not address hazard communication. Therefore, OSHA does not believe they are specific enough to justify omitting the relevant precautionary statement from labels. Label preparers can add more specific supplementary information from standards as long as it complies with paragraph C.3. For example, they may reference OSHA's flammable liquids standard (29 CFR 1910.106), which addresses the requirements for electrical equipment in workplaces that store or handle flammable liquids. OSHA requests comments on its preliminary decision not to include the conditional instruction from the GHS.
Under the HCS, a precautionary statement for gases under pressure (C.4.18) currently says "Protect from sunlight." The GHS Rev. 7 contains a conditional instruction indicating that this precautionary statement "may be omitted for gases filled in transportable gas cylinders in accordance with packing instruction P200 of the UN Recommendations on the Transport of Dangerous Goods, Model Regulations, unless those gases are subject to (slow) decomposition or polymerization, or the competent authority provides otherwise" (UN GHS, 2017, Document ID 0060). These special packaging instructions under P200 are not applicable to cylinders used in the U.S; therefore, OSHA is not proposing to add this conditional instruction to C.4.18 (UN GHS, 2017, Document ID 0060).
F. Appendix D
OSHA is proposing several changes to appendix D. These changes are being proposed to align with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060) to clarify existing requirements where stakeholders have expressed confusion, and to ensure consistency with updated scientific principles. A redline strike out version of appendix D, which reflects all of OSHA's proposed revisions, is available in the docket and on the OSHA website (OSHA HCS Redline 2020, Document ID 0222); https://www.osha.gov/dsg/hazcom/). This will allow interested parties to view all of the proposed changes in context. OSHA strongly encourages stakeholders to review that document in conjunction with the discussion of the proposed revisions below.
In the introductory section of appendix D, OSHA proposes to add a sentence stating that while each section of the SDS must contain all of the specified information, preparers of SDSs are not required to present the information in any particular order within each section. OSHA proposes this change to help clarify that while all required information must be present on the SDS, there are no mandates about the order in which the information is presented within each section. Because the information within each section can be listed in any order, OSHA does not anticipate any increased burden on SDS preparers from this change.
In section 1, Identification, OSHA is proposing revisions to clarify that the address and telephone number provided Start Printed Page 9722 on the SDS must be domestic. Although OSHA explained in a 2016 letter of interpretation that a U.S. telephone number and U.S. address are required for the SDS and label (Lee, 2016, Document ID 0090), OSHA believes it is important to codify this requirement in the text of the HCS to minimize any future confusion.
In section 2, Hazard(s) identification, OSHA is proposing to clarify where and how chemical hazard information should be presented. First, OSHA proposes to clarify that paragrah (a) must include any hazards associated with a change in the chemical's physical form under normal conditions of use, an issue the agency has addressed in several LOIs (Cawthorn, 2014, Document ID 0238; McCarthy, 2015, Document ID 0185; Fox, 2008; Document ID 0239). For example, for a chemical that poses a combustible dust hazard when processed (but not in the form in which it is shipped), the combustible dust hazard must be included in section 2(a). OSHA is also proposing a new paragraph (c) covering hazards identified under normal conditions of use that result from a chemical reaction (changing the chemical structure of the original substance or mixture). One example of such a reaction under normal conditions of use is the chemical change and subsequent physical effects of adding water to ready-mix concrete or cement, which creates additional hazards besides those present before the water is added (MST; 1995, Document ID 0253). This information is already required on the SDS (Boros, 2014, Document ID 0171), but OSHA believes that adding this language in paragraph (c) of section 2 would provide a clear and separate location for chemical manufacturers, distributors and importers to place this information. To accommodate the new material being proposed for paragraph (c), OSHA is proposing to move existing paragraphs (c) and (d) to paragraphs (d) and (e). OSHA notes that if it adopts the proposed revisions to section 2, hazards associated with chemicals as shipped, as well as hazards associated with a change in the chemical's physical form under normal conditions of use, would be presented in paragraph (a), and new hazards created by a chemical reaction under normal conditions of use would be presented in paragraph (c). OSHA believes this would sufficiently differentiates the different types of hazards presented under normal conditions of use, but welcomes stakeholder comments on this issue.
In section 3, OSHA is proposing several changes. Under the subheading "For Substances (d)" OSHA is proposing to add "(constituents)" to clarify the term "additives." OSHA intends that any individual part of an "additive" that contributes to the classification of that material needs to be listed in section 3 of the SDS. OSHA is also proposing to revise the information provided for mixtures. In addition to the information required for substances, section 3 requires the chemical name of all ingredients in a mixture that are classified as health hazards. OSHA proposes also requiring the CAS number or other unique identifier for these ingredients. CAS numbers are unique numerical identifiers assigned by the American Chemical Society (ACS) (CAS, 2020, Document ID 0173). CAS numbers are internationally recognized as being reliable and readily validated, are unique to only one compound, substance or chemical, and provide a common link between various nomenclature that may be used as descriptors for the substance or compound (UN, 2005, Document ID 0130; CAS, 2020, Document ID 0173). CAS numbers have been generated for all substances identified from the scientific literature from 1957 to the present, with some substances identified as far back as the early 1900s (CAS, 2020, Document ID 0173; UN, 2005, Document ID 0130). OSHA believes that this information provides the downstream user with important information, since it provides a unique descriptor of the chemical where the chemical identity may be ambiguous.
OSHA is proposing an additional change in section 3 to reflect the proposed revision to paragraph (i) (Trade secrets), which would allow for concentration ranges to be withheld as a trade secret. When the concentration or concentration range is withheld as a trade secret, the chemical composition range would have to be provided in accordance with the prescribed concentration ranges in paragraphs (i)(1)(iv)(A) through (M). As explained in the summary and explanation section for paragraph (i), this would create an alignment with the WHMIS under Health Canada (Canadian Gazette II, 2018, Document ID 0101).
Section 8 of the SDS includes information on exposure controls/personal protection. Section 8(a) currently requires the SDS to include the OSHA permissible exposure limit (PEL), American Conference of Governmental Industrial Hygienists (ACGIH) Threshold Limit Value (TLV), and any other exposure limit used or recommended by the chemical manufacturer, importer, or employer preparing the SDS, when available. OSHA has received questions about whether this requirement applies to individual ingredients and constituents in the mixture, and has explained that it applies to any ingredient or constituent identified in section 3 of the SDS (McVeigh, 2013, Document ID 0088). To clarify this point, OSHA is proposing to revise section 8(a) to state that it applies to all ingredients or constituents listed in section 3. OSHA notes, however, that if the ingredient or constituent does not have an OSHA PEL, ACGIH TLV or any other exposure limit used or recommend by the SDS preparer, then the ingredient or constituent would not need to be listed in section 8.
In addition, OSHA is also proposing to revise section 8 to add language indicating that SDS preparers must also include a "range" of exposure limits whenever a range is used or recommended by the chemical manufacturer, importer, or employer preparing the SDS. This revision would acknowledge new tools, such as occupational exposure banding or hazard banding methods described by NIOSH and the United Kingdom Health and Safety Executive (NIOSH, 2017, Document ID 0106; HSE, 2013, Document ID 0104). Hazard banding and occupational exposure banding provide a concentration range (band) based on toxicity and hazard information associated with a known chemical with similar properties; this range can inform appropriate risk management decisions where a specific occupational exposure limit (OEL) or permissible exposure limit (PEL) is not available or out of date (NIOSH, 2017, Document ID 0106) This type of information has been developed and validated over the last few decades and these types of exposure ranges can provide hazard information on chemicals that can help reduce risk to workers, even if limited toxicological information is available (NIOSH, 2017, Document ID 0106). As noted by NIOSH and the U.S. EPA, more than 85,000 chemicals are in commerce, with only approximately one thousand having been assessed for hazard and toxicity (either through an authoritative entity or peer-reviewed process) (NIOSH, 2017, Document ID 0106; EPA, 2016, Document ID 0058).
OSHA is proposing several updates to section 9, Physical and chemical properties. OSHA proposes to revise section 9 to align with the GHS Rev. 7 by listing the required physical and chemical properties of the chemical in the same order that appears in the GHS (UN GHS, 2017, Document ID 0060). While OSHA does not require SDS Start Printed Page 9723 preparers to list the physical and chemical properties in any particular order, the agency is proposing this change in order to simplify preparation for those chemical manufacturers that may prepare SDSs for global distribution. Other proposed changes to section 9 include: Replacing "appearance" with "physical state" and "color"; eliminating "odor threshold" and "evaporation rate" as separate required properties; adding the term "kinematic" to the property "viscosity" in order to better define the appropriate parameter to be characterized (i.e., kinematic as opposed to dynamic viscosity); and adding "particle characteristics" as a new physical property. Particle characteristics apply to solids only and the description should include the particle size (median and range) and, if available and appropriate, further properties such as size distribution (range), shape and aspect ratio, and specific surface area. Particle characteristics can be an important indicator of the potential for a solid particle to pose a hazard as particles that are less than 100 microns increase the likelihood of exposure, especially through the route of inhalation (UN GHS, 2017, Document ID 0060; UN GHS, 2016; Document ID 0143, UN GHS, 2014, Document ID 0129).
OSHA is proposing one change to section 10 of the SDS, Stability and reactivity. Section 10(c) requires preparers to include the possibility of hazardous reactions, and OSHA is proposing to clarify that this includes hazardous reactions associated with foreseeable emergencies. The proposed language is consistent with the language OSHA is proposing for paragraph (d)(1) (Hazard classification).
In addition, OSHA is proposing to revise section 11, Toxicological information, to align with the GHS Rev. 7 (UN GHS, 2017, Document ID 0060) by adding interactive effects as paragraph (e). In light of that proposed change, OSHA is proposing to move existing paragraph (e) to paragraph (f). OSHA also proposes to add a new paragraph (g), providing that when specific chemical data or information is not available, SDS preparers must indicate if alternative information is used and the method used to derive the information (e.g., where the preparer is using information from a class of chemicals rather than the exact chemical in question and using structure activity relationships (SAR) to derive the toxicological information). SAR and QSAR (quantitative SAR) are predictive tools that utilize the properties from known chemical structures and properties in relation to their biological activity to predict activities and properties of untested chemicals based on their structural similarity to tested chemicals (EPA, 2016, Document ID 0179). Read across is another predictive technique that uses information on an endpoint from a known (tested) substance to predict endpoint information from a similar (but unknown or untested) substance (ECHA, 2016, Document ID 0178). Specific, detailed examples of read across, SAR and QSAR are provided on the OECD's website for chemical safety—assessment of chemicals (OECD, 2019, Document ID 0091; EPA, 2016, Document ID 0179; ECHA, 2016, Document ID 0178). This proposed change reflects the advancement in the scientific application of computational toxicology to hazard assessment and identification and would align with the GHS Rev. 7 (Ballabio, 2018, Document ID 0128; Idakwo, 2020, Document ID 0123; OECD, Document ID 0091; Mangiatoridi, 2016, Document ID 0122; UN GHS, 2017, Document ID 0060).
Finally, OSHA is proposing to change non-mandatory section 14(f), Transport information, to read "Transport in bulk according to IMO instructions"[60] instead of "Transport in bulk (according to Annex II of MARPOL 73/78 and the IBC Code)"[61] to be consistent with text in the GHS Rev. 7 (IMSBC, 2017, Document ID 0141). This change is an update to the reference that previously only covered liquefied gases in bulk. The proposed change would provide guidance that the information in section 14 covers all bulk transport regardless of the physical form of the cargo, in accordance with IMO instruments: e.g., Annex II or Annex V of MARPOL 73/789, the IBC code10, the IMSBC[62] code and the IGC[63] code. This change would also reflect standardization of conventions for the technology and safety upgrades in the IMO (a global standard-setting authority for the safety, security and environmental performance of international shipping under the United Nations).
XVI. Authority and Signature
This document was prepared under the direction of Loren Sweatt, Principal Deputy Assistant Secretary of Labor for Occupational Safety and Health, U.S. Department of Labor, 200 Constitution Avenue NW, Washington, DC 20210. It is issued under the authority of sections 4, 6, and 8 of the Occupational Safety and Health Act of 1970 (29 U.S.C. 653, 655, 657); 5 U.S.C. 553; section 304, Clean Air Act Amendments of 1990 (Pub. L. 101-549, reprinted at 29 U.S.C.A. 655 Note); section 41, Longshore and Harbor Workers' Compensation Act (33 U.S.C. 941); section 107, Contract Work Hours and Safety Standards Act (40 U.S.C. 3704); section 1031, Housing and Community Development Act of 1992 (42 U.S.C. 4853); section 126, Superfund Amendments and Reauthorization Act of 1986, as amended (reprinted at 29 U.S.C.A. 655 Note); Secretary of Labor's Order No. 8-2020 (85 FR 58383-94); and 29 CFR part 1911.
Start List of Subjects
List of Subjects in 29 CFR Part 1910
- Chemicals
- Diseases
- Explosives
- Flammable materials
- Gases
- Hazardous substances
- Incorporation by reference
- Labeling
- Occupational safety and health
- Safety
- Signs and symbols
End List of Subjects Start Signature
Signed at Washington, DC, on December 28, 2020.
Loren Sweatt,
Principal Deputy Assistant Secretary of Labor for Occupational Safety and Health.
End Signature
Proposed Amendments
For the reasons set forth in the preamble, chapter XVII of title 29, part 1910 of the Code of Federal Regulations is proposed to be amended as follows:
Start Part
PART 1910—OCCUPATIONAL SAFETY AND HEALTH STANDARDS
End Part Start Amendment Part
1. The authority citation for part 1910 continues to read as follows:
End Amendment Part
33 U.S.C. 941; 29 U.S.C. 653, 655, 657; Secretary of Labor's Order No. 12-71 (36 FR 8754); 8-76 (41 FR 25059), 9-83 (48 FR 35736), 1-90 (55 FR 9033), 6-96 (62 FR 111), 3-2000 (65 FR 50017), 5-2002 (67 FR 65008), 5-2007 (72 FR 31160), 4-2010 (75 FR 55355), 1-2012 (77 FR 3912), or 08-2020 (85 FR 58393); 29 CFR part 1911; and 5 U.S.C. 553, as applicable.
End Authority Start Amendment Part
2. Amend § 1910.6 by:
End Amendment Part Start Amendment Part
a. Revising the last sentence of paragraph (a)(4);
End Amendment Part Start Amendment Part
b. Adding paragraphs (h)(29) and (r)(2)(vi);
End Amendment Part Start Amendment Part
c. Redesignating paragraphs (r)(4) and (5) as paragraphs (r)(6) and (7), redesignating paragraph (r)(3) as paragraph (r)(4), and adding new paragraphs (r)(3) and (r)(5);
End Amendment Part Start Amendment Part
d. Revising paragraph (bb); and
End Amendment Part Start Amendment Part
e. Adding paragraphs (cc) and (dd).
End Amendment Part
The revisions and additions read as follows:
Start Printed Page 9724
Incorporation by reference.
(a) * * *
(4) * * * For information on the availability of this material at NARA, email fedreg.legal@nara.gov or go to www.archives.gov/federal-register/cfr/ibr-locations.html.
* * * * *
(h) * * *
(29) ASTM D 4359-90 (2019), Standard Test Method for Determining Whether a Material is a Liquid or a Solid, Re-approved 2019, IBR approved for § 1910.1200.
* * * * *
(r) * * *
(2) * * *
(vi) International Organization for Standardization, ISO Central Secretariat, Chemin de Blandonnet 8 CP 401—1214 Vernier, Geneva, Switzerland; Telephone: +41 22 749 01 11; Fax: +41 22 733 34 30; Email: central@iso.org; website: https://www.iso.org/store.html.
(3) ISO 817:2014, Refrigerants—Designation and safety classification. Third Edition, June, 2014, IBR approved for appendix B to § 1910.1200.
* * * * *
(5) ISO 10156:2010, Gases and Gas Mixtures—Determination of Fire Potential and Oxidizing Ability for the Selection of Cylinder Valve Outlets, Third Edition, April, 2010, IBR approved for appendix B to § 1910.1200.
* * * * *
(bb) Except as noted, copies of the standards listed in this paragraph (bb) are available for purchase from United Nations Publications, P.O. Box 960 Herndon, VA 20172; telephone: 1-703-661-1571; fax: 1-703-996-1010; email: order@un.org.
(1) European Agreement Concerning the International Carriage of Dangerous Goods by Road (ADR), section 2.3.4 of Annex A, 2019, IBR approved for § 1910.1200. Website: https://shop.un.org/series/european-agreement-concerning-international-carriage-dangerous-goods-road-adr.
(2) UN ST/SG/AC.10/Rev.4, The UN Recommendations on the Transport of Dangerous Goods, Manual of Tests and Criteria, Fourth Revised Edition, 2003, IBR approved for appendix B to § 1910.1200. Copies available from:
(i) Bernan, 15200 NBN Way, Blue Ridge Summit, PA 17214; telephone: 1-800-865-3457; fax: 1-800-865-3450; email: customercare@bernan; website: http://www.bernan.com;
(ii) Renouf Publishing Co. Ltd., 812 Proctor Avenue, Ogdensburg, NY 13669-2205; telephone: 1-888-551-7470; Fax: 1-888-551-7471; email: orders@renoufbooks.com; website: http://www.renoufbooks.com; and
(iii) United Nations Publications, Customer Service, c/o National Book Network, 15200 NBN Way, P.O. Box 190, Blue Ridge Summit, PA 17214; telephone: 1-888-254-4286; fax: 1-800-338-4550; email: unpublications@nbnbooks.com.
(3) UN ST/SG/AC.10/30/Rev.6, The UN Recommendations on the Transport of Dangerous Goods, Manual of Tests and Criteria, Sixth Revised Edition, 2015, IBR approved for appendix B to § 1910.1200. Website: https://www.unece.org/trans/danger/publi/manual/manual_e.html.
(cc) The following material is available for purchase from International Electrotechnical Commission through ANSI, 25 West 43rd Street, 4th Floor, New York, NY 10036; telephone: 1-212-642-4963; fax: 1-212-398-0023; website: https://www.iec.ch.
(1) IEC 60079-20-1 ed. 1.0 (2010-01) Explosive atmospheres—Part 20-1: Material characteristics for gas and vapor classification—Test methods and data, IBR approved for appendix B to § 1910.1200.
(2) [Reserved]
(dd) The following material is available for purchase from German Institute for Standardization (DIN) through ANSI, 25 West 43rd Street, 4th Floor, New York, NY 10036; telephone: 1-212-642-4963; fax: 1-212-398-0023; https://din.de/en/about-standards/buy-standard.
(1) DIN 51794—Determining the ignition temperature of petroleum products, 2003, IBR approved for appendix B to § 1910.1200.
(2) [Reserved]
Start Amendment Part
3. Amend § 1910.1200:
End Amendment Part Start Amendment Part
a. By revising paragraphs (a)(1) and (b)(6)(x);
End Amendment Part Start Amendment Part
b. In paragraph (c):
End Amendment Part Start Amendment Part
i. By removing the period following the subject heading and adding a colon in its place;
End Amendment Part Start Amendment Part
ii. By adding in alphabetical order definitions for "Bulk shipment" and "Combustible dust";
End Amendment Part Start Amendment Part
iii. By revising the definition of "Exposure or exposed";
End Amendment Part Start Amendment Part
iv. By adding in alphabetical order a definition for "Gas";
End Amendment Part Start Amendment Part
v. By revising the definition of "Hazardous chemical";
End Amendment Part Start Amendment Part
vi. By adding in alphabetical order definitions for "Immediate outer package" and "Liquid";
End Amendment Part Start Amendment Part
vii. By revising the definition of "Physical hazard";
End Amendment Part Start Amendment Part
viii. By adding in alphabetical order a definition for "Physician or other licensed health are professional (PLHCP)";
End Amendment Part Start Amendment Part
ix. By removing the definition of "Pyrophoric gas"; and
End Amendment Part Start Amendment Part
x. By adding in alphabetical order definitions for "Released for shipment" and "Solid";
End Amendment Part Start Amendment Part
c. By revising paragraphs (d)(1), (e)(4), and (f)(1), (5), and (11);
End Amendment Part Start Amendment Part
d. By adding paragraph (f)(12);
End Amendment Part Start Amendment Part
e. By revising paragraphs (g)(2) introductory text, (g)(10), (i)(1) and (2), (i)(3) introductory text, and (j); and
End Amendment Part Start Amendment Part
f. By revising appendices A through D.
End Amendment Part
The revisions and additions read as follows:
Hazard communication.
(a) * * *
(1) The purpose of this section is to ensure that the hazards of all chemicals produced or imported are classified, and that information concerning the classified hazards is transmitted to employers and employees. The requirements of this section are intended to be consistent with the provisions of the United Nations Globally Harmonized System of Classification and Labeling of Chemicals (GHS), Revision 7. The transmittal of information is to be accomplished by means of comprehensive hazard communication programs, which are to include container labeling and other forms of warning, safety data sheets and employee training.
* * * * *
(b) * * *
(6) * * *
(x) Nuisance particulates where the chemical manufacturer or importer can establish that they do not pose any physical hazard, health hazard, or other hazards covered under this section;
* * * * *
(c) * * *
Bulk shipment means any hazardous chemical transported where the mode of transportation (vehicle) comprises the immediate container (i.e., contained in tanker truck, rail car, or intermodal container).
* * * * *
Combustible dust means finely divided solid particles of a substance or mixture that are liable to catch fire or explode on ignition when dispersed in air or other oxidizing media.
* * * * *
Exposure or exposed means that an employee is subjected in the course of employment to a hazardous chemical, and includes potential (e.g., accidental or possible) exposure. "Subjected" in terms of health hazards includes any route of entry (e.g., inhalation, ingestion, skin contact or absorption).
* * * * *
Gas means a substance which—at 122 °F (50 °C) has a vapor pressure greater than 43.51 PSI (300 kPa) (absolute); or Start Printed Page 9725 is completely gaseous at 68 °F (20 °C) at a standard pressure of 14.69 PSI (101.3 kPa).
* * * * *
Hazardous chemical means any chemical which is classified as a physical hazard or a health hazard, a simple asphyxiant, combustible dust, or hazard not otherwise classified.
* * * * *
Immediate outer package means the first package enclosing the container of hazardous chemical.
* * * * *
Liquid means a substance or mixture which at 122 °F (50 °C) has a vapor pressure of not more than 43.51 PSI (300 kPa (3 bar)), which is not completely gaseous at 68 °F (20 °C) and at a standard pressure of 101.3 kPa, and which has a melting point or initial melting point of 68 °F (20 °C) or less at a standard pressure of 14.69 PSI (101.3 kPa). A viscous substance or mixture for which a specific melting point cannot be determined shall be subjected to ASTM D 4359-90 (2019) (Standard Test Method for Determining Whether a Material Is a Liquid or a Solid) (incorporated by reference; see § 1910.6); or to the test for determining fluidity (penetrometer test) prescribed in the European Agreement Concerning the International Carriage of Dangerous Goods by Road (ADR), section 2.3.4 of Annex A (2019) (incorporated by reference; see § 1910.6).
* * * * *
Physical hazard means a chemical that is classified as posing one of the following hazardous effects: Explosive; flammable (gases, liquids, or solids); aerosols; oxidizer (liquid, solid or gas); self-reactive; pyrophoric (liquid or solid); self-heating; organic peroxide; corrosive to metal; gas under pressure; in contact with water emits flammable gas; or desensitized explosive. The criteria for determining whether a chemical is classified as a physical hazard are detailed in appendix B to this section.
Physician or other licensed health care professional (PLHCP) means an individual whose legally permitted scope of practice (i.e., license, registration, or certification) allows the individual to independently provide or be delegated the responsibility to provide some or all of the health care services referenced in paragraph (i) of this section.
* * * * *
Released for shipment means a chemical that has been packaged and labeled in the manner in which it will be distributed or sold.
* * * * *
Solid means a substance or mixture which does not meet the definitions of liquid or gas.
* * * * *
(d) * * *
(1) Chemical manufacturers and importers shall evaluate chemicals produced in their workplaces or imported by them to classify the chemicals in accordance with this section. For each chemical, the chemical manufacturer or importer shall determine the hazard classes, and where appropriate, the category of each class that apply to the chemical being classified under normal conditions of use and foreseeable emergencies. The hazard classification shall include any hazards associated with a change in the chemical's physical form or resulting from a reaction with other chemicals under normal conditions of use. Employers are not required to classify chemicals unless they choose not to rely on the classification performed by the chemical manufacturer or importer for the chemical to satisfy this paragraph (d)(1).
* * * * *
(e) * * *
(4) The employer shall make the written hazard communication program available, upon request, to employees, their designated representatives, the Assistant Secretary and the Director, in accordance with the requirements of § 1910.1020(e).
* * * * *
(f) * * *
(1) Labels on shipped containers. The chemical manufacturer, importer, or distributor shall ensure that each container of hazardous chemicals leaving the workplace is labeled, tagged or marked. Hazards not otherwise classified and hazards resulting from a reaction with other chemicals under normal conditions of use do not have to be addressed on the container. Where the chemical manufacturer, importer, or distributor is required to label, tag or mark the following shall be provided:
(i) Product identifier;
(ii) Signal word;
(iii) Hazard statement(s);
(iv) Pictogram(s);
(v) Precautionary statement(s);
(vi) Name, address, and telephone number of the chemical manufacturer, importer, or other responsible party; and
(vii) Date chemical is released for shipment.
* * * * *
(5) Transportation. (i) Chemical manufacturers, importers, or distributors shall ensure that each container of hazardous chemicals leaving the workplace is labeled, tagged, or marked in accordance with this section in a manner which does not conflict with the requirements of the Hazardous Materials Transportation Act (49 U.S.C. 1801 et seq.) and regulations issued under that Act by the Department of Transportation.
(ii) The label for bulk shipments of hazardous chemicals may be on the immediate container or may be transmitted with the shipping papers, bills of lading, or other technological or electronic means so that it is immediately available to workers in printed form on the receiving end of shipment.
(iii) Where a pictogram required by the Department of Transportation under title 49 of the Code of Federal Regulations appears on the label for a shipped container, the pictogram specified in appendix C.4 of this section for the same hazard is not required on the label.
* * * * *
(11) Release for shipment. Chemical manufacturers, importers, distributors, or employers who become newly aware of any significant information regarding the hazards of a chemical shall revise the labels for the chemical within six months of becoming aware of the new information, and shall ensure that labels on containers of hazardous chemicals shipped after that time contain the new information. Chemicals that have been released for shipment and are awaiting future distribution need not be relabeled; however, the chemical manufacturer or importer must provide the updated label for each individual container with each shipment. If the chemical is not currently produced or imported, the chemical manufacturer, importer, distributor, or employer shall add the information to the label before the chemical is shipped or introduced into the workplace again.
(12) Small container labelling. (i) This paragraph (f)(12) applies where the chemical manufacturer, importer, or distributor can demonstrate that it is not feasible to use pull-out labels, fold-back labels, or tags containing the full label information required by paragraph (f)(1) of this section.
(ii) For a container less than or equal to 100 ml capacity, the chemical manufacturer, importer, or distributor must include, at a minimum, the following information on the label of the container:
(A) Product identifier;
(B) Pictogram(s);
(C) Signal word;
(D) Chemical manufacturer's name and phone number; and
(E) A statement that the full label information for the hazardous chemical Start Printed Page 9726 is provided on the immediate outer package.
(iii) For a container less than or equal to 3 ml capacity, where the chemical manufacturer, importer, or distributor can demonstrate that any label interferes with the normal use of the container, no label is required, but the container must bear, at a minimum, the product identifier.
(iv) For all small containers covered by paragraph (f)(12)(ii) or (iii) of this section, the immediate outer package must include:
(A) The full label information required by paragraph (f)(1) of this section for each hazardous chemical in the immediate outer package. The label must not be removed or defaced, as required by paragraph (f)(9) of this section.
(B) A statement that the small container(s) inside must be stored in the immediate outer package bearing the complete label when not in use.
(g) * * *
(2) The chemical manufacturer or importer shall ensure that the safety data sheet is in English (although the employer may maintain copies in other languages as well), and includes at least the following section numbers and headings, and associated information under each heading, in the order listed (See appendix D to this section for the specific content of each section of the safety data sheet):
* * * * *
(10) Safety data sheets may be kept in any form, including as operating procedures, and may be stored in such a way to cover groups of hazardous chemicals in a work area where it may be more appropriate to address the hazards of a process rather than individual hazardous chemicals. However, the employer shall ensure that in all cases the required information is provided for each hazardous chemical, and is readily accessible during each work shift to employees when they are in their work area(s).
* * * * *
(i) * * *
(1) The chemical manufacturer, importer, or employer may withhold the specific chemical identity, including the chemical name, other specific identification of a hazardous chemical, or the exact percentage (concentration) or concentration range of the substance in a mixture, from section 3 of the safety data sheet, provided that:
(i) The claim that the information withheld is a trade secret can be supported;
(ii) Information contained in the safety data sheet concerning the properties and effects of the hazardous chemical is disclosed;
(iii) The safety data sheet indicates that the specific chemical identity and/or concentration or concentration range of composition is being withheld as a trade secret;
(iv) If the concentration or concentration range is being claimed as a trade secret then the safety data sheet provides the ingredient's concentration as one of the prescribed ranges in paragraphs (i)(1)(iv)(A) through (M) of this section.
(A) From 0.1% to 1%;
(B) From 0.5% to 1.5%;
(C) From 1% to 5%;
(D) From 3% to 7%;
(E) From 5% to 10%;
(F) From 7% to 13%;
(G) From 10% to 30%;
(H) From 15% to 40%;
(I) From 30% to 60%;
(J) From 45% to 70%;
(K) From 60% to 80%;
(L) From 65% to 85%; and
(M) From 80% to 100%.
(v) The prescribed concentration range used must be the narrowest range possible. If the exact concentration range falls between 0.1% and 30% and does not fit entirely into one of the prescribed concentration, a single range created by the combination of two applicable consecutive ranges (e.g., between (i)(1)(iv)(A) and (G)) may be disclosed instead, provided that the combined concentration range does not include any range that falls entirely outside the exact concentration range in which the ingredient is present.
(vi) The specific chemical identity and exact concentration or concentration range is made available to health professionals, employees, and designated representatives in accordance with the applicable provisions of this paragraph (i).
(2) Where a treating PLHCP determines that a medical emergency exists and the specific chemical identity and/or specific concentration or concentration range of a hazardous chemical is necessary for emergency or first-aid treatment, the chemical manufacturer, importer, or employer shall immediately disclose the specific chemical identity or percentage composition of a trade secret chemical to that treating PLHCP, regardless of the existence of a written statement of need or a confidentiality agreement. The chemical manufacturer, importer, or employer may require a written statement of need and confidentiality agreement, in accordance with the provisions of paragraphs (i)(3) and (4) of this section, as soon as circumstances permit.
(3) In non-emergency situations, a chemical manufacturer, importer, or employer shall, upon request, disclose a specific chemical identity or exact concentration or concentration range, otherwise permitted to be withheld under paragraph (i)(1) of this section, to a health professional (e.g., PLHCP, industrial hygienist, toxicologist, or epidemiologist) providing medical or other occupational health services to exposed employee(s), and to employees or designated representatives, if:
* * * * *
(j) Dates. (1) This section is effective [DATE 60 DAYS AFTER DATE OF PUBLICATION OF FINAL RULE IN THE FEDERAL REGISTER].
(2) Chemical manufacturers, importers, and distributors evaluating substances shall be in compliance with all modified provisions of this section no later than [DATE ONE YEAR AFTER EFFECTIVE DATE OF FINAL RULE].
(3) Chemical manufacturers, importers, and distributors evaluating mixtures shall be in compliance with all modified provisions of this section no later than 24 months after [DATE TWO YEARS AFTER EFFECTIVE DATE OF FINAL RULE].
* * * * *
Appendix A to § 1910.1200—Health Hazard Criteria (Mandatory)
A.0 General Classification Considerations
A.0.1 Classification
A.0.1.1 The term "hazard classification" is used to indicate that only the intrinsic hazardous properties of chemicals are considered. Hazard classification incorporates three steps:
(a) Identification of relevant data regarding the hazards of a chemical;
(b) Subsequent review of those data to ascertain the hazards associated with the chemical;
(c) Determination of whether the chemical will be classified as hazardous and the degree of hazard.
A.0.1.2 For many hazard classes, the criteria are semi-quantitative or qualitative and expert judgment is required to interpret the data for classification purposes.
A.0.1.3 Where impurities, additives or individual constituents of a substance or mixture have been identified and are themselves classified, they should be taken into account during classification if they exceed the cut-off value/concentration limit for a given hazard class.
A.0.2 Available Data, Test Methods and Test Data Quality
A.0.2.1 There is no requirement for testing chemicals.
A.0.2.2 The criteria for determining health hazards are test method neutral, i.e., they do not specify particular test methods, as long as the methods are scientifically validated. Start Printed Page 9727
A.0.2.3 The term "scientifically validated" refers to the process by which the reliability and the relevance of a procedure are established for a particular purpose. Any test that determines hazardous properties, which is conducted according to recognized scientific principles, can be used for purposes of a hazard determination for health hazards. Test conditions need to be standardized so that the results are reproducible with a given substance, and the standardized test yields "valid" data for defining the hazard class of concern.
A.0.2.4 Existing test data are acceptable for classifying chemicals, although expert judgment also may be needed for classification purposes.
A.0.2.5 The effect of a chemical on biological systems is influenced, by the physico-chemical properties of the substance and/or ingredients of the mixture and the way in which ingredient substances are biologically available. A chemical need not be classified when it can be shown by conclusive experimental data from scientifically validated test methods that the chemical is not biologically available.
A.0.2.6 For classification purposes, epidemiological data and experience on the effects of chemicals on humans (e.g., occupational data, data from accident databases) shall be taken into account in the evaluation of human health hazards of a chemical.
A.0.3 Classification Based on Weight of Evidence
A.0.3.1 For some hazard classes, classification results directly when the data satisfy the criteria. For others, classification of a chemical shall be determined on the basis of the total weight of evidence using expert judgment. This means that all available information bearing on the classification of hazard shall be considered together, including the results of valid in vitro tests, relevant animal data, and human experience such as epidemiological and clinical studies and well-documented case reports and observations.
A.0.3.2 The quality and consistency of the data shall be considered. Information on chemicals related to the material being classified shall be considered as appropriate, as well as site of action and mechanism or mode of action study results. Both positive and negative results shall be considered together in a single weight-of-evidence determination.
A.0.3.3 Positive effects which are consistent with the criteria for classification, whether seen in humans or animals, shall normally justify classification. Where evidence is available from both humans and animals and there is a conflict between the findings, the quality and reliability of the evidence from both sources shall be evaluated in order to resolve the question of classification. Reliable, good quality human data shall generally have precedence over other data. However, even well-designed and conducted epidemiological studies may lack a sufficient number of subjects to detect relatively rare but still significant effects, or to assess potentially confounding factors. Therefore, positive results from well-conducted animal studies are not necessarily negated by the lack of positive human experience but require an assessment of the robustness, quality and statistical power of both the human and animal data.
A.0.3.4 Route of exposure, mechanistic information, and metabolism studies are pertinent to determining the relevance of an effect in humans. When such information raises doubt about relevance in humans, a lower classification may be warranted. When there is scientific evidence demonstrating that the mechanism or mode of action is not relevant to humans, the chemical should not be classified.
A.0.3.5 Both positive and negative results are considered together in the weight of evidence determination. However, a single positive study performed according to good scientific principles and with statistically and biologically significant positive results may justify classification.
A.0.4 Considerations for the Classification of Mixtures
A.0.4.1 Except as provided in A.0.4.2, the process of classification of mixtures is based on the following sequence:
(a) Where test data are available for the complete mixture, the classification of the mixture will always be based on those data;
(b) Where test data are not available for the mixture itself, the bridging principles designated in each health hazard chapter of this appendix shall be considered for classification of the mixture;
(c) If test data are not available for the mixture itself, and the available information is not sufficient to allow application of the above-mentioned bridging principles, then the method(s) described in each chapter for estimating the hazards based on the information known will be applied to classify the mixture (e.g., application of cut-off values/concentration limits).
A.0.4.2 An exception to the above order or precedence is made for Carcinogenicity, Germ Cell Mutagenicity, and Reproductive Toxicity. For these three hazard classes, mixtures shall be classified based upon information on the ingredient substances, unless on a case-by-case basis, justification can be provided for classifying based upon the mixture as a whole. See chapters A.5, A.6, and A.7 for further information on case-by-case bases.
A.0.4.3 Use of cut-off values/concentration limits
A.0.4.3.1 When classifying an untested mixture based on the hazards of its ingredients, cut-off values/concentration limits for the classified ingredients of the mixture are used for several hazard classes. While the adopted cut-off values/concentration limits adequately identify the hazard for most mixtures, there may be some that contain hazardous ingredients at lower concentrations than the specified cut-off values/concentration limits that still pose an identifiable hazard. There may also be cases where the cut-off value/concentration limit is considerably lower than the established non-hazardous level for an ingredient.
A.0.4.3.2 If the classifier has information that the hazard of an ingredient will be evident (i.e., it presents a health risk) below the specified cut-off value/concentration limit, the mixture containing that ingredient shall be classified accordingly.
A.0.4.3.3 In exceptional cases, conclusive data may demonstrate that the hazard of an ingredient will not be evident (i.e., it does not present a health risk) when present at a level above the specified cut-off value/concentration limit(s). In these cases the mixture may be classified according to those data. The data must exclude the possibility that the ingredient will behave in the mixture in a manner that would increase the hazard over that of the pure substance. Furthermore, the mixture must not contain ingredients that would affect that determination.
A.0.4.4 Synergistic or antagonistic effects
When performing an assessment in accordance with these requirements, the evaluator must take into account all available information about the potential occurrence of synergistic effects among the ingredients of the mixture. Lowering classification of a mixture to a less hazardous category on the basis of antagonistic effects may be done only if the determination is supported by sufficient data.
A.0.5 Bridging principles for the classification of mixtures where test data are not available for the complete mixture
A.0.5.1 Where the mixture itself has not been tested to determine its toxicity, but there are sufficient data on both the individual ingredients and similar tested mixtures to adequately characterize the hazards of the mixture, these data shall be used in accordance with the following bridging principles, subject to any specific provisions for mixtures for each hazard class. These principles ensure that the classification process uses the available data to the greatest extent possible in characterizing the hazards of the mixture.
A.0.5.1.1 Dilution
For mixtures classified in accordance with A.1 through A.10 of this appendix, if a tested mixture is diluted with a diluent that has an equivalent or lower toxicity classification than the least toxic original ingredient, and which is not expected to affect the toxicity of other ingredients, then:
(a) The new diluted mixture shall be classified as equivalent to the original tested mixture; or
(b) For classification of acute toxicity in accordance with A.1 of this appendix, paragraph A.1.3.6 (the additivity formula) shall be applied.
A.0.5.1.2 Batching
For mixtures classified in accordance with A.1 through A.10 of this appendix, the toxicity of a tested production batch of a mixture can be assumed to be substantially equivalent to that of another untested production batch of the same mixture, when produced by or under the control of the same chemical manufacturer, unless there is reason to believe there is significant variation such that the toxicity of the untested batch has changed. If the latter occurs, a new classification is necessary.
A.0.5.1.3 Concentration of mixtures
For mixtures classified in accordance with A.1, A.2, A.3, A.4, A.8, A.9, or A.10 of this appendix, if a tested mixture is classified in Category 1, and the concentration of the Start Printed Page 9728 ingredients of the tested mixture that are in Category 1 is increased, the resulting untested mixture shall be classified in Category 1.
A.0.5.1.4 Interpolation within one hazard category
For mixtures classified in accordance with A.1, A.2, A.3, A.4, A.8, A.9, or A.10 of this appendix, for three mixtures (A, B and C) with identical ingredients, where mixtures A and B have been tested and are in the same hazard category, and where untested mixture C has the same toxicologically active ingredients as mixtures A and B but has concentrations of toxicologically active ingredients intermediate to the concentrations in mixtures A and B, then mixture C is assumed to be in the same hazard category as A and B.
A.0.5.1.5 Substantially similar mixtures
For mixtures classified in accordance with A.1 through A.10 of this appendix, given the following set of conditions:
(a) Where there are two mixtures: (i) A + B; (ii) C + B;
(b) The concentration of ingredient B is essentially the same in both mixtures;
(c) The concentration of ingredient A in mixture (i) equals that of ingredient C in mixture (ii);
(d) And data on toxicity for A and C are available and substantially equivalent; i.e., they are in the same hazard category and are not expected to affect the toxicity of B; then
If mixture (i) or (ii) is already classified based on test data, the other mixture can be assigned the same hazard category.
A.0.5.1.6 Aerosols
For mixtures classified in accordance with A.1, A.2, A.3, A.4, A.8, or A.9 of this appendix, an aerosol form of a mixture shall be classified in the same hazard category as the tested, non-aerosolized form of the mixture, provided the added propellant does not affect the toxicity of the mixture when spraying.
A.1 Acute Toxicity
A.1.1 Definition
Acute toxicity refers to serious adverse health effects (i.e., lethality) occurring after a single or short-term oral, dermal, or inhalation exposure to a substance or mixture.
A.1.2 Classification Criteria for Substances
A.1.2.1 Substances can be allocated to one of four hazard categories based on acute toxicity by the oral, dermal or inhalation route according to the numeric cut-off criteria as shown in Table A.1.1. Acute toxicity values are expressed as (approximate) LD50 (oral, dermal) or LC50 (inhalation) values or as acute toxicity estimates (ATE). While some in vivo methods determine LD50/LC50 values directly, other newer in vivo methods (e.g., using fewer animals) consider other indicators of acute toxicity, such as significant clinical signs of toxicity, which are used by reference to assign the hazard category. See the footnotes following Table A.1.1 for further explanation on the application of these values.
Start Printed Page 9729

A.1.2.3 The preferred test species for evaluation of acute toxicity by the oral and inhalation routes is the rat, while the rat or rabbit are preferred for evaluation of acute dermal toxicity. Test data already generated for the classification of chemicals under Start Printed Page 9730 existing systems should be accepted when reclassifying these chemicals under the harmonized system. When experimental data for acute toxicity are available in several animal species, scientific judgment should be used in selecting the most appropriate LD50 value from among scientifically validated tests. In cases where data from human experience (i.e., occupational data, data from accident databases, epidemiology studies, clinical reports) is also available, it should be considered in a weight of evidence approach consistent with the principles described in A.0.3.
A.1.2.4 In addition to classification for inhalation toxicity, if data are available that indicates that the mechanism of toxicity was corrosivity of the substance or mixture, the classifier must consider if the chemical is corrosive to the respiratory tract. Corrosion of the respiratory tract is defined as destruction of the respiratory tract tissue after a single, limited period of exposure analogous to skin corrosion; this includes destruction of the mucosa. The corrosivity evaluation could be based on expert judgment using such evidence as: Human and animal experience, existing (in vitro) data, pH values, information from similar substances or any other pertinent data.
A.1.2.4.1 If the classifier determines the chemical is corrosive to the respiratory tract and data are available that indicate that the effect leads to lethality, then the chemical must be labelled with the hazard statement "corrosive to the respiratory tract."
A.1.2.4.2 If the classifier determines the chemical is corrosive to the respiratory tract and the effect does not lead to lethality, then the chemical must be addressed in the Specific Target Organ Toxicity hazard classes (see A.8 and A.9).
A.1.3 Classification Criteria for Mixtures
A.1.3.1 The approach to classification of mixtures for acute toxicity is tiered, and is dependent upon the amount of information available for the mixture itself and for its ingredients. The flow chart of Figure A.1.1 indicates the process that must be followed:

Start Printed Page 9731
A.1.3.2 Classification of mixtures for acute toxicity may be carried out for each route of exposure, but is only required for one route of exposure as long as this route is followed (estimated or tested) for all ingredients and there is no relevant evidence to suggest acute toxicity by multiple routes. When there is relevant evidence of acute toxicity by multiple routes of exposure, classification is to be conducted for all appropriate routes of exposure. All available information shall be considered. The pictogram and signal word used shall reflect the most severe hazard category; and all relevant hazard statements shall be used.
A.1.3.3 For purposes of classifying the hazards of mixtures in the tiered approach:
(a) The "relevant ingredients" of a mixture are those which are present in concentrations ≥1% (weight/weight for solids, liquids, dusts, mists and vapors and volume/volume for gases). If there is reason to suspect that an ingredient present at a concentration <1% will affect classification of the mixture for acute toxicity, that ingredient shall also be considered relevant. Consideration of ingredients present at a concentration <1% is particularly important when classifying untested mixtures which contain ingredients that are classified in Category 1 and Category 2;
(b) Where a classified mixture is used as an ingredient of another mixture, the actual or derived acute toxicity estimate (ATE) for that mixture is used when calculating the classification of the new mixture using the formulas in A.1.3.6.1 and A.1.3.6.2.4.
(c) If the converted acute toxicity point estimates for all ingredients of a mixture are within the same category, then the mixture should be classified in that category.
(d) When only range data (or acute toxicity hazard category information) are available for ingredients in a mixture, they may be converted to point estimates in accordance with Table A.1.2 when calculating the classification of the new mixture using the formulas in A.1.3.6.1 and A.1.3.6.2.4.
A.1.3.4 Classification of Mixtures Where Acute Toxicity Test Data Are Available for the Complete Mixture
Where the mixture itself has been tested to determine its acute toxicity, it is classified according to the same criteria as those used for substances, presented in Table A.1.1. If test data for the mixture are not available, the procedures presented below must be followed.
A.1.3.5 Classification of Mixtures Where Acute Toxicity Test Data Are Not Available for the Complete Mixture: Bridging Principles
A.1.3.5.1 Where the mixture itself has not been tested to determine its acute toxicity, but there are sufficient data on both the individual ingredients and similar tested mixtures to adequately characterize the hazards of the mixture, these data will be used in accordance with the following bridging principles as found in paragraph A.0.5 of this appendix: Dilution, Batching, Concentration of mixtures, Interpolation within one hazard category, Substantially similar mixtures, and Aerosols.
A.1.3.6 Classification of Mixtures Based on Ingredients of the Mixture (Additivity Formula)
A.1.3.6.1 Data available for all ingredients.
The acute toxicity estimate (ATE) of ingredients is considered as follows:
(a) *Include ingredients with a known acute toxicity, which fall into any of the acute toxicity hazard categories, or have an oral or dermal LD50 greater than 2,000 but less than or equal to 5,000 mg/kg body weight (or the equivalent dose for inhalation);
(b) Ignore ingredients that are presumed not acutely toxic (e.g., water, sugar);
(c) Ignore ingredients if the data available are from a limit dose test (at the upper threshold for Category 4 for the appropriate route of exposure as provided in Table A.1.1) and do not show acute toxicity.
Ingredients that fall within the scope of this paragraph are considered to be ingredients with a known acute toxicity estimate (ATE). See note (b) to Table A.1.1 and paragraph A.1.3.3 for appropriate application of available data to the equation below, and paragraph A.1.3.6.2.4.
The ATE of the mixture is determined by calculation from the ATE values for all relevant ingredients according to the following formula below for oral, dermal or inhalation toxicity:

where:
Ci = concentration of ingredient i
n ingredients and i is running from 1 to n
ATEi = acute toxicity estimate of ingredient i.
A.1.3.6.2 Data are not available for one or more ingredients of the mixture
A.1.3.6.2.1 Where an ATE is not available for an individual ingredient of the mixture, but available information provides a derived conversion value, the formula in A.1.3.6.1 may be applied. This information may include evaluation of:
(a) Extrapolation between oral, dermal and inhalation acute toxicity estimates. Such an evaluation requires appropriate pharmacodynamic and pharmacokinetic data;
(b) Evidence from human exposure that indicates toxic effects but does not provide lethal dose data;
(c) Evidence from any other toxicity tests/assays available on the substance that indicates toxic acute effects but does not necessarily provide lethal dose data; or
(d) Data from closely analogous substances using structure/activity relationships.
A.1.3.6.2.2 This approach requires substantial supplemental technical information, and a highly trained and experienced expert, to reliably estimate acute toxicity. If sufficient information is not available to reliably estimate acute toxicity, proceed to the provisions of A.1.3.6.2.4.
A.1.3.6.2.3 In the event that an ingredient with unknown acute toxicity is used in a mixture at a concentration ≥1%, and the mixture has not been classified based on testing of the mixture as a whole, the mixture cannot be attributed a definitive acute toxicity estimate. In this situation the mixture is classified based on the known ingredients only. Note: A statement that × percent of the mixture consists of ingredient(s) of unknown acute (oral/dermal/inhalation) toxicity is required on the label and safety data sheet in such cases; see appendix C to this section, Allocation of Label Elements and appendix D to this section, Safety Data Sheets.)
A.1.3.6.2.4 If the total concentration of the relevant ingredient(s) with unknown acute toxicity is ≤10% then the formula presented in A.1.3.6.1 must be used. If the total concentration of the relevant ingredient(s) with unknown acute toxicity is >10%, the formula presented in A.1.3.6.1 is corrected to adjust for the percentage of the unknown ingredient(s) as follows:

A.2 Skin Corrosion/Irritation
A.2.1 Definitions and General Considerations
A.2.1.1Skin corrosion refers to the production of irreversible damage to the skin; namely, visible necrosis through the epidermis and into the dermis occurring after exposure to a substance or mixture.
Skin irritation refers to the production of reversible damage to the skin occurring after exposure to a substance or mixture.
A.2.1.2 Skin corrosion/irritation shall be classified using a tiered approach as detailed in figure A.2.1. Emphasis shall be placed upon existing human data (See A.0.2.6), followed by existing animal data, followed by in vitro data and then other sources of information. Classification results directly when the data satisfy the criteria in this section. In case the criteria cannot be directly applied, classification of a substance or a mixture is made on the basis of the total weight of evidence (See A.0.3.1). This means that all available information bearing on the determination of skin corrosion/irritation is considered together, including the results of appropriate scientifically validated in-vitro tests, relevant animal data, and human data Start Printed Page 9732 such as epidemiological and clinical studies and well-documented case reports and observations.
A.2.2 Classification Criteria for Substances
Substances shall be allocated to one of the following categories within this hazard class:
(a) Category 1 (skin corrosion)
This category may be further divided into up to three sub-categories (1A, 1B and 1C)
(b) Category 2 (skin irritation)
A.2.2.1 Classification Based on Standard Animal Test Data
A.2.2.1.1 Skin Corrosion
A.2.2.1.2 A substance is corrosive to the skin when it produces destruction of skin tissue, namely, visible necrosis through the epidermis and into the dermis, in at least one tested animal after exposure up to a 4-hour duration.
A.2.2.1.3 Three sub-categories of Category 1 are provided in Table A.2.1, all of which shall be regulated as Category 1.
| Criteria | |
|---|---|
| Category 1 | Destruction of skin tissue, namely, visible necrosis through the epidermis and into the dermis, in at least one tested animal after exposure ≤4 h. |
| Sub-category 1A | Corrosive responses in at least one animal following exposure ≤3 min during an observation period ≤1 h. |
| Sub-category 1B | Corrosive responses in at least one animal following exposure >3 min and ≤1 h and observations ≤14 days. |
| Sub-category 1C | Corrosive responses in at least one animal after exposures >1 h and ≤4 h and observations ≤14 days. |
| a The use of human data is discussed in A.2.3. | |
A.2.2.2 Skin Irritation
A.2.2.2.1 A single irritant category (Category 2) is presented in the Table A.2.2. A substance is irritant to skin when it produces reversible damage to the skin following its application for up to 4 hours.
The major criterion for the irritant category is that at least 2 tested animals have a mean score of ≥2.3 ≤4.0.
A.2.2.2.2 An irritation category (Category 2) is provided that:
(a) Recognizes that some test substances may lead to effects which persist throughout the length of the test; and
(b) acknowledges that animal responses in a test may be variable.
A.2.2.2.3 Reversibility of skin lesions is another consideration in evaluating irritant responses. When inflammation persists to the end of the observation period in two or more test animals, taking into consideration alopecia (limited area), hyperkeratosis, hyperplasia and scaling, then a chemical should be considered to be an irritant.
A.2.2.2.4 Animal irritant responses within a test can be quite variable, as they are with corrosion. A separate irritant criterion accommodates cases when there is a significant irritant response but less than the mean score criterion for a positive test. For example, a substance might be designated as an irritant if at least 1 of 3 tested animals shows a very elevated mean score throughout the study, including lesions persisting at the end of an observation period of normally 14 days. Other responses could also fulfill this criterion. However, it should be ascertained that the responses are the result of chemical exposure. Addition of this criterion increases the sensitivity of the classification system.
| Criteria | |
|---|---|
| Irritant (Category 2) | (1) Mean score of ≥2.3 ≤4.0 for erythema/eschar or for edema in at least 2 of 3 tested animals from gradings at 24, 48 and 72 hours after patch removal or, if reactions are delayed, from grades on 3 consecutive days after the onset of skin reactions; or |
| (2) Inflammation that persists to the end of the observation period normally 14 days in at least 2 animals, particularly taking into account alopecia (limited area), hyperkeratosis, hyperplasia, and scaling; or | |
| (3) In some cases where there is pronounced variability of response among animals, with very definite positive effects related to chemical exposure in a single animal but less than the criteria above. | |
| a Grading criteria are understood as described in OECD Test Guideline 404. | |
A.2.3 Classification in a Tiered Approach
A.2.3.1 A tiered approach to the evaluation of initial information shall be used (Figure A.2.1) recognizing that not all elements may be relevant.
A.2.3.2 Existing human and animal data including information from single or repeated exposure should be the first line of evaluation, as they give information directly relevant to effects on the skin.
A.2.3.3 Acute dermal toxicity data may be used for classification. If a substance is highly toxic by the dermal route, a skin corrosion/irritation study may not be practicable since the amount of test substance to be applied would considerably exceed the toxic dose and, consequently, would result in the death of the animals. When observations are made of skin corrosion/irritation in acute toxicity studies and are observed up through the limit dose, these data may be used for classification provided that the dilutions used and species tested are equivalent. Solid substances (powders) may become corrosive or irritant when moistened or in contact with moist skin or mucous membranes.
A.2.3.4 In vitro alternatives that have been scientifically validated shall be used to make classification decisions.
A.2.3.5 Likewise, pH extremes like ≤2 and ≥11.5 may indicate skin effects, especially when associated with significant acid/alkaline reserve (buffering capacity). Generally, such substances are expected to produce significant effects on the skin. In the absence of any other information, a substance is considered corrosive (Skin Category 1) if it has a pH ≤2 or a pH ≥11.5. However, if consideration of acid/alkaline reserve suggests the substance or mixture may not be corrosive despite the low or high pH value, this needs to be confirmed by other data, preferably data from an appropriate validated in vitro test.
A.2.3.6 In some cases sufficient information may be available from structurally related substances to make classification decisions.
A.2.3.7 The tiered approach explains how to organize existing information on a substance and to make a weight of evidence decision about hazard assessment and hazard classification (ideally without conducting new animal tests). Although information might be gained from the evaluation of single parameters within a tier, consideration should be given to the totality of existing information and making an overall weight of evidence determination. This is especially true when there is conflict in information available on some parameters.
Start Printed Page 9733

Start Printed Page 9734

Start Printed Page 9735
A.2.4 Classification Criteria for Mixtures
A.2.4.1 Classification of Mixtures When Data Are Available for the Complete Mixture
A.2.4.1.1 The mixture shall be classified using the criteria for substances, taking into account the tiered approach to evaluate data for this hazard class (as illustrated in Figure A.2.1).
A.2.4.1.2 When considering testing of the mixture, classifiers must use a tiered approach as included in the criteria for classification of substances for skin corrosion and irritation to help ensure an accurate classification, as well as to avoid unnecessary animal testing. In the absence of any other information, a mixture is considered corrosive (Skin Category 1) if it has a pH ≤2 or a pH ≥11.5. However, if consideration of acid/alkaline reserve suggests the mixture may not be corrosive despite the low or high pH value, then further evaluation may be necessary.
A.2.4.2 Classification of Mixtures When Data Are Not Available for the Complete Mixture: Bridging Principles
A.2.4.2.1 Where the mixture itself has not been tested to determine its skin corrosion/irritation potential, but there are sufficient data on both the individual ingredients and similar tested mixtures to adequately characterize the hazards of the mixture, these data will be used in accordance with the following bridging principles, as found in paragraph A.0.5 of this appendix: Dilution, Batching, Concentration of mixtures, Interpolation within one hazard category, Substantially similar mixtures, and Aerosols.
A.2.4.3 Classification of Mixtures When Data Are Available for All Ingredients or Only for Some Ingredients of the Mixture
A.2.4.3.1 In order to make use of all available data for purposes of classifying the skin corrosion/irritation hazards of mixtures, the following assumption has been made and is applied where appropriate in the tiered approach:
The "relevant ingredients" of a mixture are those which are present in concentrations ≥1% (weight/weight for solids, liquids, dusts, mists and vapors and volume/volume for gases). If the classifier has reason to suspect that an ingredient present at a concentration <1% will affect classification of the mixture for skin corrosion/irritation, that ingredient shall also be considered relevant.
A.2.4.3.2 In general, the approach to classification of mixtures as corrosive or irritant to the skin when data are available on the ingredients, but not on the mixture as a whole, is based on the theory of additivity, such that each corrosive or irritant ingredient contributes to the overall corrosive or irritant properties of the mixture in proportion to its potency and concentration. A weighting factor of 10 is used for corrosive ingredients when they are present at a concentration below the concentration limit for classification with Category 1, but are at a concentration that will contribute to the classification of the mixture as an irritant. The mixture is classified as corrosive or irritant when the sum of the concentrations of such ingredients exceeds a cut-off value/concentration limit.
A.2.4.3.3 Table A.2.3 below provides the cut-off value/concentration limits to be used to determine if the mixture is considered to be corrosive or irritant to the skin.
A.2.4.3.4 Particular care shall be taken when classifying certain types of chemicals such as acids and bases, inorganic salts, aldehydes, phenols, and surfactants. The approach explained in A.2.4.3.1 and A.2.4.3.2 might not work given that many of such substances are corrosive or irritant at concentrations <1%. For mixtures containing strong acids or bases the pH should be used as classification criteria since pH will be a better indicator of corrosion than the concentration limits in Table A.2.3. A mixture containing corrosive or irritant ingredients that cannot be classified based on the additivity approach shown in Table A.2.3, due to chemical characteristics that make this approach unworkable, should be classified as skin corrosion Category 1 if it contains ≥1% of a corrosive ingredient and as skin irritation Category 2 when it contains ≥3% of an irritant ingredient. Classification of mixtures with ingredients for which the approach in Table A.2.3 does not apply is summarized in Table A.2.4 below.
A.2.4.3.5 On occasion, reliable data may show that the skin corrosion/irritation of an ingredient will not be evident when present at a level above the generic cut-off values/concentration limits mentioned in Tables A.2.3 and A.2.4. In these cases the mixture could be classified according to those data (See Use of cut-off values/concentration limits, paragraph A.0.4.3 of this appendix).
A.2.4.3.6 If there are data showing that (an) ingredient(s) may be corrosive or irritant to skin at a concentration of <1% (corrosive) or <3% (irritant), the mixture shall be classified accordingly (See Use of cut-off values/concentration limits, paragraph A.0.4.3 of this appendix).
| Sum of ingredients classified as: | Concentration triggering classification of a mixture as: | |
|---|---|---|
| Skin corrosive | Skin irritant | |
| Category 1 | Category 2 | |
| Skin Category 1 | ≥5% | ≥1% but <5% |
| Skin Category 2 | ≥10% | |
| (10 × Skin Category 1) + Skin Category 2 | ≥10% | |
| Note: Where data are available and the sub-categories of skin Category 1 (corrosive) are used, the sum of all ingredients of a mixture classified as sub-category 1A, 1B or 1C respectively, must each be ≥5% in order to classify the mixture as either skin sub-category 1A, 1B or 1C. Where the sum of 1A ingredients is <5% but the sum of 1A + 1B ingredients is ≥5%, the mixturemust be classified as sub-category 1B. Similarly, where the sum of 1A + 1B ingredients is <5% but the sum of 1A + 1B + 1C ingredients is ≥5% the mixture must be classified as sub-category 1C. Where at least one relevant ingredient in a mixture is classified as Category 1 without sub-categorization, the mixture must be classified as Category 1 without sub-categorization if the sum of all ingredients corrosive to skin is ≥5%. | ||
| Ingredient: | Concentration: | Mixture classified as: Skin |
|---|---|---|
| Acid with pH ≤2 | ≥1% | Category 1. |
| Base with pH ≥11.5 | ≥1% | Category 1. |
| Other corrosive (Category 1) ingredient | ≥1% | Category 1. |
| Other irritant (Category 2) ingredient, including acids and bases | ≥3% | Category 2. |
Start Printed Page 9736
A.3 Serious Eye Damage/Eye Irritation
A.3.1 Definitions and General Considerations
A.3.1.1Serious eye damage refers to the production of tissue damage in the eye, or serious physical decay of vision, which is not fully reversible, occurring after exposure of the eye to a substance or mixture.
Eye irritation refers to the production of changes in the eye, which are fully reversible, occurring after exposure of the eye to a substance or mixture.
A.3.1.2 Serious eye damage/eye irritation shall be classified using a tiered approach as detailed in Figure A.3.1. Emphasis shall be placed upon existing human data (See A.0.2.6), followed by existing animal data, followed by in vitro data and then other sources of information. Classification results directly when the data satisfy the criteria in this section. In case the criteria cannot be directly applied, classification of a substance or a mixture is made on the basis of the total weight of evidence (See A.0.3.1). This means that all available information bearing on the determination of serious eye damage/eye irritation is considered together, including the results of appropriate scientifically validated in vitro tests, relevant animal data, and human data such as epidemiological and clinical studies and well-documented case reports and observations.
A.3.2 Classification Criteria for Substances
Substances are allocated to one of the categories within this hazard class, Category 1 (serious eye damage) or Category 2 (eye irritation), as follows:
(a) Category 1 (serious eye damage/irreversible effects on the eye): Substances that have the potential to seriously damage the eyes (see Table A.3.1).
(b) Category 2 (eye irritation/reversible effects on the eye): Substances that have the potential to induce reversible eye irritation (see Table A.3.2).
A.3.2.1Classification based on standard animal test data.
A.3.2.1.1 Serious eye damage (Category 1)/Irreversible effects on the eye.
A single hazard category is provided in Table A.3.1, for substances that have the potential to seriously damage the eyes. Category 1, irreversible effects on the eye, includes the criteria listed below. These observations include animals with grade 4 cornea lesions and other severe reactions (e.g., destruction of cornea) observed at any time during the test, as well as persistent corneal opacity, discoloration of the cornea by a dye substance, adhesion, pannus, and interference with the function of the iris or other effects that impair sight. In this context, persistent lesions are considered those which are not fully reversible within an observation period of normally 21 days. Category 1 also contains substances fulfilling the criteria of corneal opacity ≥3 and/or iritis >1.5 observed in at least 2 of 3 tested animals detected in a Draize eye test with rabbits, because severe lesions like these usually do not reverse within a 21-day observation period.
| Criteria | |
|---|---|
| Category 1: Serious eye damage/Irreversible effects on the eye | A substance that produces: (a) In at least one animal effects on the cornea, iris or conjunctiva that are not expected to reverse or have not fully reversed within an observation period of normally 21 days; and/or (b) in at least 2 of 3 tested animals, a positive response of: |
| (i) Corneal opacity ≥3; and/or | |
| (ii) iritis >1.5; | |
| calculated as the mean scores following grading at 24, 48 and 72 hours after instillation of the test material. | |
| a Grading criteria are understood as described in OECD Test Guideline 405. | |
A.3.2.2 Eye irritation (Category 2)/Reversible effects on the eye.
A.3.2.2.1 A single Category 2 is provided in Table A.3.2 for substances that have the potential to induce reversible eye irritation.
When data are available, substances may be classified into Category 2A and Category 2B:
(a) For substances inducing eye irritant effects reversing within an observation time of normally 21 days, Category 2A applies.
(b) For substances inducing eye irritant effects reversing within an observation time of 7 days, Category 2B applies.
When a substance is classified as Category 2, without further categorization, the classification criteria are the same as those for 2A.
A.3.2.3 For those substances where there is pronounced variability among animal responses, this information may be taken into account in determining the classification.
| Criteria | |
|---|---|
| Substances that have the potential to induce reversible eye irritation. | |
| Category 2/2A | Substances that produce in at least 2 of 3 tested animals a positive response of: |
| (a) corneal opacity ≥1; and/or | |
| (b) iritis ≥1; and/or | |
| (c) conjunctival redness ≥2; and/or | |
| (d) conjunctival edema (chemosis) ≥2 | |
| calculated as the mean scores following grading at 24, 48 and 72 hours after instillation of the test material, and which fully reverses within an observation period of normally 21 days. | |
| Category 2B | Within Category 2A an eye irritant is considered mildly irritating to eyes (Category 2B) when the effects listed above are fully reversible within 7 days of observation. |
| a Grading criteria are understood as described in OECD Test Guideline 405. | |
A.3.3 Classification in a Tiered Approach
A.3.3.1 A tiered approach to the evaluation of initial information shall be used where applicable, recognizing that all elements may not be relevant in certain cases (Figure A.3.1).
A.3.3.2 Existing human and animal data should be the first line of analysis, as they give information directly relevant to effects on the eye. Possible skin corrosion shall be evaluated prior to consideration of any testing for serious eye damage/eye irritation in order to avoid testing for local effects on eyes with skin corrosive substances.
A.3.3.3In vitro alternatives that have been scientifically validated and accepted shall be used to make classification decisions.
A.3.3.4 Likewise, pH extremes like ≤2 and ≥11.5, may indicate serious eye damage, especially when associated with significant acid/alkaline reserve (buffering capacity). Generally, such substances are expected to produce significant effects on the eyes. In the absence of any other information, a substance is considered to cause serious eye damage (Category 1) if it has a pH ≤2 or ≥11.5. Start Printed Page 9737 However, if consideration of acid/alkaline reserve suggests the substance may not cause serious eye damage despite the low or high pH value, this needs needs to be confirmed by other data, preferably by data from an appropriate validated in vitro test.
A.3.3.5 In some cases sufficient information may be available from structurally related substances to make classification decisions.
A.3.3.6 The tiered approach explains how to organize existing information and to make a weight-of-evidence decision about hazard assessment and hazard classification (ideally without conducting new animal tests). Animal testing with corrosive substances should be avoided wherever possible. Although information might be gained from the evaluation of single parameters within a tier, consideration should be given to the totality of existing information and making an overall weight of evidence determination. This is especially true when there is conflict in information available on some parameters.
Start Printed Page 9738

Start Printed Page 9739

Start Printed Page 9740

A.3.4 Classification Criteria for Mixtures
A.3.4.1 Classification of Mixtures When Data Are Available for the Complete Mixture
A.3.4.1.1 The mixture will be classified using the criteria for substances, and taking into account the tiered approach to evaluate data for this hazard class (as illustrated in Figure A.3.1).
A.3.4.1.2 When considering testing of the mixture, chemical manufacturers shall use a tiered approach as included in the criteria for classification of substances for skin corrosion and serious eye damage and eye irritation to help ensure an accurate classification, as well as to avoid unnecessary animal testing. In the absence of any other information, a mixture is considered to cause serious eye damage (Category 1) if it has a pH ≤2 or ≥11.5. However, if consideration of acid/alkaline reserve suggests the mixture may not have the potential to cause serious eye damage despite the low or high pH value, then further evaluation may be necessary.
A.3.4.2 Classification of Mixtures When Data Are Not Available for the Complete Mixture: Bridging Principles
A.3.4.2.1 Where the mixture itself has not been tested to determine its skin corrosivity or potential to cause serious eye damage or eye irritation, but there are sufficient data on both the individual ingredients and similar tested mixtures to adequately characterize the hazards of the mixture, these data will be used in accordance with the following bridging principles, as found in paragraph A.0.5 of this appendix: Dilution, Batching, Concentration of mixtures, Interpolation within one hazard category, Substantially similar mixtures, and Aerosols.
A.3.4.3 Classification of Mixtures When Data Are Available for All Ingredients or Only for Some Ingredients of the Mixture
A.3.4.3.1 For purposes of classifying the serious eye damage/eye irritation hazards of mixtures in the tiered approach:
The "relevant ingredients" of a mixture are those which are present in concentrations ≥1% (weight/weight for solids, liquids, dusts, mists and vapors and volume/volume for gases). If the classifier has reason to suspect that an ingredient present at a concentration <1% will affect classification of the mixture for serious eye damage/eye irritation, that ingredient shall also be considered relevant.
A.3.4.3.2 In general, the approach to classification of mixtures as seriously damaging to the eye or eye irritant when data are available on the ingredients, but not on the mixture as a whole, is based on the theory of additivity, such that each skin corrosive or serious eye damage/eye irritant ingredient contributes to the overall serious eye damage/eye irritation properties of the mixture in proportion to its potency and concentration. A weighting factor of 10 is used for skin corrosive and serious eye damaging ingredients when they are present at a concentration below the concentration limit for classification with Category 1, but are at a concentration that will contribute to the classification of the mixture as serious eye damaging/eye irritant. The mixture is classified as seriously damaging to the eye or eye irritant when the sum of the concentrations of such ingredients exceeds a threshold cut-off value/concentration limit.
A.3.4.3.3 Table A.3.3 provides the cut-off value/concentration limits to be used to determine if the mixture must be classified as seriously damaging to the eye or an eye irritant.
A.3.4.3.4 Particular care must be taken when classifying certain types of chemicals such as acids and bases, inorganic salts, aldehydes, phenols, and surfactants. The approach explained in A.3.4.3.1 and A.3.4.3.2 might not work given that many of such substances are seriously damaging to the eye/eye irritating at concentrations <1%. For mixtures containing strong acids or bases, the pH should be used as classification criteria (See A.3.4.1) since pH will be a better indicator of serious eye damage (subject to consideration of acid/alkali reserve) than the concentration limits of Table A.3.3. A mixture containing skin corrosive or serious eye damaging/eye irritating ingredients that cannot be classified based on the additivity approach applied in Table A.3.3 due to chemical characteristics that make this approach unworkable, should be classified as serious eye damage (Category 1) if it contains ≥1% of a skin corrosive or serious eye damaging ingredient and as Eye Irritation (Category 2) when it contains ≥3% of an eye irritant ingredient. Classification of mixtures with ingredients for which the approach in Table A.3.3 does not apply is summarized in Table A.3.4.
A.3.4.3.5 On occasion, reliable data may show that the irreversible/reversible eye effects of an ingredient will not be evident when present at a level above the generic cut-off values/concentration limits mentioned in Tables A.3.3 and A.3.4. In these cases the mixture could be classified according to those data (See also A.0.4.3 Use of cut-off values/concentration limits"). On occasion, when it is expected that the skin corrosion/irritation or the reversible/irreversible eye effects of an ingredient will not be evident when present at a level above the generic concentration/cut-off levels mentioned in Tables A.3.3 and A.3.4, testing of the mixture may be considered. In those cases, the tiered weight of evidence approach should be applied as referred to in section A.3.3, Figure A.3.1 and explained in detail in this chapter.
A.3.4.3.6 If there are data showing that (an) ingredient(s) may be corrosive to the skin or seriously damaging to the eye/eye irritating at a concentration of <1% (corrosive to the skin or seriously damaging to the eye) or <3% (eye irritant), the mixture shall be classified accordingly (See also paragraph A.0.4.3, Use of cut-off values/concentration limits). Start Printed Page 9741
| Sum of ingredients classified as | Concentration triggering classification of a mixture as | |
|---|---|---|
| Serious eye damage | Eye irritation | |
| Category 1 | Category 2/2A | |
| Skin corrosion (Category 1) + Serious eye damage (Category 1)a | ≥3% | ≥1% but <3%. |
| Eye irritation (Category 2) | b ≥10%. | |
| 10 × (Skin corrosion (Category 1) + Serious eye damage (Category 1))a + Eye irritation (Category 2) | ≥10%. | |
| Notes: | ||
| a If an ingredient is classified as both skin Category 1 and eye Category 1 its concentration is considered only once in the calculation. | ||
| b A mixture may be classified as Eye Irritation Category 2B in cases when all relevant ingredients are classified as Eye Irritation Category 2B. | ||
| Ingredient | Concentration (%) | Mixture classified as: |
|---|---|---|
| Acid with pH ≤2 | ≥1 | Serious eye damage (Category 1). |
| Base with pH ≥11.5 | ≥1 | Serious eye damage (Category 1). |
| Other skin corrosive or serious eye damage (Category 1) ingredients | ≥1 | Serious eye damage (Category 1). |
| Other eye irritant (Category 2) ingredients | ≥3 | Eye irritation (Category 2). |
A.4 Respiratory or Skin Sensitization
A.4.1 Definitions and General Considerations
A.4.1.1 Respiratory sensitization refers to hypersensitivity of the airways occurring after inhalation of a substance or mixture.
Skin sensitization refers to an allergic response occurring after skin contact with a substance or mixture.
A.4.1.2 For the purpose of this chapter, sensitization includes two phases: The first phase is induction of specialized immunological memory in an individual by exposure to an allergen. The second phase is elicitation, i.e., production of a cell-mediated or antibody-mediated allergic response by exposure of a sensitized individual to an allergen.
A.4.1.3 For respiratory sensitization, the pattern of induction followed by elicitation phases is shared in common with skin sensitization. For skin sensitization, an induction phase is required in which the immune system learns to react; clinical symptoms can then arise when subsequent exposure is sufficient to elicit a visible skin reaction (elicitation phase). As a consequence, predictive tests usually follow this pattern in which there is an induction phase, the response to which is measured by a standardized elicitation phase, typically involving a patch test. The local lymph node assay is the exception, directly measuring the induction response. Evidence of skin sensitization in humans normally is assessed by a diagnostic patch test.
A.4.1.4 Usually, for both skin and respiratory sensitization, lower levels are necessary for elicitation than are required for induction.
A.4.1.5 The hazard class "respiratory or skin sensitization" is differentiated into:
(a) Respiratory sensitization; and
(b) Skin sensitization.
A.4.2 Classification Criteria for Substances
A.4.2.1 Respiratory Sensitizers
A.4.2.1.1 Hazard categories
A.4.2.1.1.1 Effects seen in either humans or animals will normally justify classification in a weight of evidence approach for respiratory sensitizers. Substances may be allocated to one of the two sub-categories 1A or 1B using a weight of evidence approach in accordance with the criteria given in Table A.4.1 and on the basis of reliable and good quality evidence from human cases or epidemiological studies and/or observations from appropriate studies in experimental animals.
A.4.2.1.1.2 Where data are not sufficient for sub-categorization, respiratory sensitizers shall be classified in Category 1.
| Category 1: | Respiratory sensitizer |
|---|---|
| A substance is classified as a respiratory sensitizer: (a) If there is evidence in humans that the substance can lead to specific respiratory hypersensitivity and/or (b) if there are positive results from an appropriate animal test.1 | |
| Sub-category 1A: | Substances showing a high frequency of occurrence in humans; or a probability of occurrence of a high sensitization rate in humans based on animal or other tests.1 Severity of reaction may also be considered. |
| Sub-category 1B: | Substances showing a low to moderate frequency of occurrence in humans; or a probability of occurrence of a low to moderate sensitization rate in humans based on animal or other tests.1 Severity of reaction may also be considered. |
A.4.2.1.2 Human evidence
A.4.2.1.2.1 Evidence that a substance can lead to specific respiratory hypersensitivity will normally be based on human experience. In this context, hypersensitivity is normally seen as asthma, but other hypersensitivity reactions such as rhinitis/conjunctivitis and alveolitis are also considered. The condition will have the clinical character of an allergic reaction. However, immunological mechanisms do not have to be demonstrated. Start Printed Page 9742
A.4.2.1.2.2 When considering the human evidence, it is necessary that in addition to the evidence from the cases, the following be taken into account:
(a) The size of the population exposed;
(b) The extent of exposure.
A.4.2.1.2.3 The evidence referred to above could be:
(a) Clinical history and data from appropriate lung function tests related to exposure to the substance, confirmed by other supportive evidence which may include:
(i) In vivo immunological test (e.g., skin prick test);
(ii) In vitro immunological test (e.g., serological analysis);
(iii) Studies that may indicate other specific hypersensitivity reactions where immunological mechanisms of action have not been proven, e.g., repeated low-level irritation, pharmacologically mediated effects;
(iv) A chemical structure related to substances known to cause respiratory hypersensitivity;
(b) Data from positive bronchial challenge tests with the substance conducted according to accepted guidelines for the determination of a specific hypersensitivity reaction.
A.4.2.1.2.4 Clinical history should include both medical and occupational history to determine a relationship between exposure to a specific substance and development of respiratory hypersensitivity. Relevant information includes aggravating factors both in the home and workplace, the onset and progress of the disease, family history and medical history of the patient in question. The medical history should also include a note of other allergic or airway disorders from childhood and smoking history.
A.4.2.1.2.5 The results of positive bronchial challenge tests are considered to provide sufficient evidence for classification on their own. It is, however, recognized that in practice many of the examinations listed above will already have been carried out.
A.4.2.1.3 Animal studies
A.4.2.1.3.1 Data from appropriate animal studies[2] which may be indicative of the potential of a substance to cause sensitization by inhalation in humans[3] may include:
(a) Measurements of Immunoglobulin E (IgE) and other specific immunological parameters, for example in mice.
(b) Specific pulmonary responses in guinea pigs.
A.4.2.2 Skin Sensitizers
A.4.2.2.1 Hazard categories
A.4.2.2.1.1 Effects seen in either humans or animals will normally justify classification in a weight of evidence approach for skin sensitizers. Substances may be allocated to one of the two sub-categories 1A or 1B using a weight of evidence approach in accordance with the criteria given in Table A.4.2 and on the basis of reliable and good quality evidence from human cases or epidemiological studies and/or observations from appropriate studies in experimental animals according to the guidance values provided in A.4.2.2.2.1 and A.4.2.2.3.2 for sub-category 1A and in A.4.2.2.2.2 and A.4.2.2.3.3 for sub-category 1B.
A.4.2.2.1.2 Where data are not sufficient for sub-categorization, skin sensitizers shall be classified in Category 1.
| Category 1: | Skin sensitizer |
|---|---|
| A substance is classified as a skin sensitizer: (a) If there is evidence in humans that the substance can lead to sensitization by skin contact in a substantial number of persons, or (b) if there are positive results from an appropriate animal test. | |
| Sub-category 1A: | Substances showing a high frequency of occurrence in humans and/or a high potency in animals can be presumed to have the potential to produce significant sensitization in humans. Severity of reaction may also be considered. |
| Sub-category 1B: | Substances showing a low to moderate frequency of occurrence in humans and/or a low to moderate potency in animals can be presumed to have the potential to produce sensitization in humans. Severity of reaction may also be considered. |
A.4.2.2.2 Human evidence
A.4.2.2.2.1 Human evidence for sub-category 1A may include:
(a) Positive responses at ≤500 µg/cm2 (Human Repeat Insult Patch Test (HRIPT), Human Maximization Test (HMT)—induction threshold);
(b) Diagnostic patch test data where there is a relatively high and substantial incidence of reactions in a defined population in relation to relatively low exposure;
(c) Other epidemiological evidence where there is a relatively high and substantial incidence of allergic contact dermatitis in relation to relatively low exposure.
A.4.2.2.2.2 Human evidence for sub-category 1B may include:
(a) Positive responses at >500 µg/cm2 (HRIPT, HMT—induction threshold);
(b) Diagnostic patch test data where there is a relatively low but substantial incidence of reactions in a defined population in relation to relatively high exposure;
(c) Other epidemiological evidence where there is a relatively low but substantial incidence of allergic contact dermatitis in relation to relatively high exposure.
A.4.2.2.3 Animal studies
A.4.2.2.3.1 For Category 1, when an adjuvant type test method for skin sensitization is used, a response of at least 30% of the animals is considered as positive. For a non-adjuvant Guinea pig test method a response of at least 15% of the animals is considered positive. For Category 1, a stimulation index of three or more is considered a positive response in the local lymph node assay.[4]
A.4.2.2.3.2 Animal test results for sub-category 1A can include data with values indicated in Table A.4.3 below:
| Assay | Criteria |
|---|---|
| Local lymph node assay | EC3 value ≤2%. |
| Guinea pig maximization test | ≥30% responding at ≤0.1% intradermal induction dose or ≥60% responding at >0.1% to ≤1% intradermal induction dose. |
| Start Printed Page 9743 | |
| Buehler assay | ≥15% responding at ≤0.2% topical induction dose or ≥60% responding at >0.2% to ≤20% topical induction dose. |
| Note: EC3 refers to the estimated concentration of test chemical required to induce a stimulation index of 3 in the local lymph node assay. | |
A.4.2.2.3.3 Animal test results for sub-category 1B can include data with values indicated in the following Table A.4.4:
| Assay | Criteria |
|---|---|
| Local lymph node assay | EC3 value >2%. |
| Guinea pig maximization test | ≥30% to <60% responding at >0.1% to ≤1% intradermal induction dose or ≥30% responding at >1% intradermal induction dose. |
| Buehler assay | ≥15% to <60% responding at >0.2% to ≤20% topical induction dose or ≥15% responding at >20% topical induction dose. |
| Note: EC3 refers to the estimated concentration of test chemical required to induce a stimulation index of 3 in the local lymph node assay. | |
A.4.2.2.4 Specific considerations
A.4.2.2.4.1 For classification of a substance, evidence shall include one or more of the following using a weight of evidence approach:
(a) Positive data from patch testing, normally obtained in more than one dermatology clinic;
(b) Epidemiological studies showing allergic contact dermatitis caused by the substance. Situations in which a high proportion of those exposed exhibit characteristic symptoms are to be looked at with special concern, even if the number of cases is small;
(c) Positive data from appropriate animal studies;
(d) Positive data from experimental studies in humans (See paragraph A.0.2.6 of this appendix);
(e) Well documented episodes of allergic contact dermatitis, normally obtained in more than one dermatology clinic;
(f) Severity of reaction.
A.4.2.2.4.2 Evidence from animal studies is usually much more reliable than evidence from human exposure. However, in cases where evidence is available from both sources, and there is conflict between the results, the quality and reliability of the evidence from both sources must be assessed in order to resolve the question of classification on a case-by-case basis. Normally, human data are not generated in controlled experiments with volunteers for the purpose of hazard classification but rather as part of risk assessment to confirm lack of effects seen in animal tests. Consequently, positive human data on skin sensitization are usually derived from case-control or other, less defined studies. Evaluation of human data must, therefore, be carried out with caution as the frequency of cases reflect, in addition to the inherent properties of the substances, factors such as the exposure situation, bioavailability, individual predisposition and preventive measures taken. Negative human data should not normally be used to negate positive results from animal studies. For both animal and human data, consideration should be given to the impact of vehicle.
A.4.2.2.4.3 If none of the above-mentioned conditions are met, the substance need not be classified as a skin sensitizer. However, a combination of two or more indicators of skin sensitization, as listed below, may alter the decision. This shall be considered on a case-by-case basis.
(a) Isolated episodes of allergic contact dermatitis;
(b) Epidemiological studies of limited power, e.g., where chance, bias or confounders have not been ruled out fully with reasonable confidence;
(c) Data from animal tests, performed according to existing guidelines, which do not meet the criteria for a positive result described in A.4.2.2.3, but which are sufficiently close to the limit to be considered significant;
(d) Positive data from non-standard methods;
(e) Positive results from close structural analogues.
A.4.2.2.4.4 Immunological contact urticaria
A.4.2.2.4.4.1 Substances meeting the criteria for classification as respiratory sensitizers may, in addition, cause immunological contact urticaria. Consideration shall be given to classifying these substances as skin sensitizers.
A.4.2.2.4.4.2 Substances which cause immunological contact urticaria without meeting the criteria for respiratory sensitizers shall be considered for classification as skin sensitizers.
A.4.2.2.4.4.3 There is no recognized animal model available to identify substances which cause immunological contact urticaria. Therefore, classification will normally be based on human evidence, similar to that for skin sensitization.
A.4.3 Classification Criteria for Mixtures
A.4.3.1 Classification of Mixtures When Data Are Available for the Complete Mixture
When reliable and good quality evidence, as described in the criteria for substances, from human experience or appropriate studies in experimental animals, is available for the mixture, then the mixture shall be classified by weight of evidence evaluation of these data. Care must be exercised in evaluating data on mixtures that the dose used does not render the results inconclusive.
A.4.3.2 Classification of Mixtures When Data Are Not Available for the Complete Mixture: Bridging Principles
A.4.3.2.1 Where the mixture itself has not been tested to determine its sensitizing properties, but there are sufficient data on both the individual ingredients and similar tested mixtures to adequately characterize the hazards of the mixture, these data will be used in accordance with the following agreed bridging principles as found in paragraph A.0.5 of this appendix: Dilution, Batching, Concentration of mixtures, Interpolation within one hazard category/subcategory, Substantially similar mixtures, and Aerosols.
A.4.3.3 Classification of Mixtures When Data Are Available for All Ingredients or Only for Some Ingredients of the Mixture
The mixture shall be classified as a respiratory or skin sensitizer when at least one ingredient has been classified as a respiratory or skin sensitizer and is present at or above the appropriate cut-off value/concentration limit for the specific endpoint as shown in Table A.4.5. Start Printed Page 9744
| Ingredient classified as: | Cut-off values/concentration limits triggering classification of a mixture as: | ||
|---|---|---|---|
| Respiratory sensitizer Category 1 | Skin sensitizer Category 1 | ||
| Solid/Liquid (%) | Gas | All physical states (%) | |
| Respiratory Sensitizer: Category 1 | ≥0.1 | ≥0.1 | |
| Respiratory Sensitizer: Sub-category 1A | ≥0.1 | ≥0.1 | |
| Respiratory Sensitizer: Sub-category 1B | ≥1.0 | ≥0.2 | |
| Skin Sensitizer: Category 1 | ≥0.1 | ||
| Skin Sensitizer: Sub-category 1A | ≥0.1 | ||
| Skin Sensitizer: Sub-category 1B | ≥1.0 | ||
A.5 Germ Cell Mutagenicity
A.5.1 Definitions and General Considerations
A.5.1.1 Germ cell mutagenicity refers to heritable gene mutations, including heritable structural and numerical chromosome aberrations in germ cells occurring after exposure to a substance or mixture.
A.5.1.2 A mutation is defined as a permanent change in the amount or structure of the genetic material in a cell. The term mutation applies both to heritable genetic changes that may be manifested at the phenotypic level and to the underlying DNA modifications when known (including, for example, specific base pair changes and chromosomal translocations). The term mutagenic and mutagen will be used for agents giving rise to an increased occurrence of mutations in populations of cells and/or organisms.
A.5.1.3 The more general terms genotoxic and genotoxicity apply to agents or processes which alter the structure, information content, or segregation of DNA, including those which cause DNA damage by interfering with normal replication processes, or which in a non-physiological manner (temporarily) alter its replication. Genotoxicity test results are usually taken as indicators for mutagenic effects.
A.5.1.4 This hazard class is primarily concerned with chemicals that may cause mutations in the germ cells of humans that can be transmitted to the progeny. However, mutagenicity/genotoxicity tests in vitro and in mammalian somatic cells in vivo are also considered in classifying substances and mixtures within this hazard class.
A.5.2 Classification Criteria for Substances
A.5.2.1 The classification system provides for two different categories of germ cell mutagens to accommodate the weight of evidence available. The two-category system is described in the Figure A.5.1.
| CATEGORY 1: Substances known to induce heritable mutations or to be regarded as if they induce heritable mutations in the germ cells of humans. |
| Category 1A: Substances known to induce heritable mutations in germ cells of humans. Positive evidence from human epidemiological studies. |
| Category 1B: Substances which should be regarded as if they induce heritable mutations in the germ cells of humans: |
| (a) Positive result(s) from in vivo heritable germ cell mutagenicity tests in mammals; or |
| (b) Positive result(s) from in vivo somatic cell mutagenicity tests in mammals, in combination with some evidence that the substance has potential to cause mutations to germ cells. This supporting evidence may, for example, be derived from mutagenicity/genotoxicity tests in germ cells in vivo, or by demonstrating the ability of the substance or its metabolite(s) to interact with the genetic material of germ cells; or |
| (c) Positive results from tests showing mutagenic effects in the germ cells of humans, without demonstration of transmission to progeny; for example, an increase in the frequency of aneuploidy in sperm cells of exposed people. |
| CATEGORY 2: Substances which cause concern for humans owing to the possibility that they may induce heritable mutations in the germ cells of humans. |
| Positive evidence obtained from experiments in mammals and/or in some cases from in vitro experiments, obtained from: |
| (a) Somatic cell mutagenicity tests in vivo, in mammals; or |
| (b) Other in vivo somatic cell genotoxicity tests which are supported by positive results from in vitro mutagenicity assays. |
| Note: Substances which are positive in in vitro mammalian mutagenicity assays, and which also show chemical structure activity relationship to known germ cell mutagens, should be considered for classification as Category 2 mutagens. |
A.5.2.2 Specific considerations for classification of substances as germ cell mutagens:
A.5.2.2.1 To arrive at a classification, test results are considered from experiments determining mutagenic and/or genotoxic effects in germ and/or somatic cells of exposed animals. Mutagenic and/or genotoxic effects determined in in vitro tests shall also be considered.
A.5.2.2.2 The system is hazard based, classifying chemicals on the basis of their intrinsic ability to induce mutations in germ cells. The scheme is, therefore, not meant for the (quantitative) risk assessment of chemical substances.
A.5.2.2.3 Classification for heritable effects in human germ cells is made on the basis of scientifically validated tests. Evaluation of the test results shall be done using expert judgment and all the available evidence shall be weighed for classification.
A.5.2.2.4 The classification of substances shall be based on the total weight of evidence available, using expert judgment. In those instances where a single well-conducted test is used for classification, it shall provide clear and unambiguously positive results. The relevance of the route of exposure used in the study of the substance compared to the route of human exposure should also be taken into account.
A.5.3 Classification Criteria for Mixtures[5]
A.5.3.1 Classification of Mixtures When Data Are Available for All Ingredients or Only for Some Ingredients of the Mixture
A.5.3.1.1 Classification of mixtures shall be based on the available test data for the Start Printed Page 9745 individual ingredients of the mixture using cut-off values/concentration limits for the ingredients classified as germ cell mutagens.
A.5.3.1.2 The mixture will be classified as a mutagen when at least one ingredient has been classified as a Category 1A, Category 1B or Category 2 mutagen and is present at or above the appropriate cut-off value/concentration limit as shown in Table A.5.1 below for Category 1 and 2 respectively.
| Ingredient classified as: | Cut-off/concentration limits triggering classification of a mixture as: | |
|---|---|---|
| Category 1 mutagen | Category 2 mutagen | |
| Category 1A/B mutagen | ≥0.1% | |
| Category 2 mutagen | ≥1.0% | |
| Note: The cut-off values/concentration limits in the table above apply to solids and liquids (w/w units) as well as gases (v/v units). | ||
A.5.3.2 Classification of Mixtures When Data Are Available for the Mixture Itself
The classification may be modified on a case-by-case basis based on the available test data for the mixture as a whole. In such cases, the test results for the mixture as a whole must be shown to be conclusive taking into account dose and other factors such as duration, observations and analysis (e.g., statistical analysis, test sensitivity) of germ cell mutagenicity test systems.
A.5.3.3 Classification of Mixtures When Data Are Not Available for the Complete Mixture: Bridging Principles
A.5.3.3.1 Where the mixture itself has not been tested to determine its germ cell mutagenicity hazard, but there are sufficient data on both the individual ingredients and similar tested mixtures to adequately characterize the hazards of the mixture, these data will be used in accordance with the following bridging principles as found in paragraph A.0.5 of this appendix: Dilution, Batching, and Substantially similar mixtures.
A.5.4 Examples of Scientifically Validated Test Methods
A.5.4.1 Examples of in vivo heritable germ cell mutagenicity tests are:
(a) Rodent dominant lethal mutation test (OECD 478)
(b) Mouse heritable translocation assay (OECD 485)
(c) Mouse specific locus test
A.5.4.2 Examples of in vivo somatic cell mutagenicity tests are:
(a) Mammalian bone marrow chromosome aberration test (OECD 475)
(b) Mammalian erythrocyte micronucleus test (OECD 474)
A.5.4.3 Examples of mutagenicity/genotoxicity tests in germ cells are:
(a) Mutagenicity tests:
(i) Mammalian spermatogonial chromosome aberration test (OECD 483)
(ii) Spermatid micronucleus assay
(b) Genotoxicity tests:
(i) Sister chromatid exchange analysis in spermatogonia
(ii) Unscheduled DNA synthesis test (UDS) in testicular cells
A.5.4.4 Examples of genotoxicity tests in somatic cells are:
(a) Liver Unscheduled DNA Synthesis (UDS) in vivo (OECD 486)
(b) Mammalian bone marrow Sister Chromatid Exchanges (SCE)
A.5.4.5 Examples of in vitro mutagenicity tests are:
(a) In vitro mammalian chromosome aberration test (OECD 473)
(b) In vitro mammalian cell gene mutation test (OECD 476)
(c) Bacterial reverse mutation tests (OECD 471)
A.5.4.6 As new, scientifically validated tests arise, these may also be used in the total weight of evidence to be considered.
A.6 Carcinogenicity
A.6.1 Definitions
Carcinogenicity refers to the induction of cancer or an increase in the incidence of cancer occurring after exposure to a substance or mixture. Substances and mixtures which have induced benign and malignant tumors in well-performed experimental studies on animals are considered also to be presumed or suspected human carcinogens unless there is strong evidence that the mechanism of tumor formation is not relevant for humans.
Classification of a substance or mixture as posing a carcinogenic hazard is based on its inherent properties and does not provide information on the level of the human cancer risk which the use of the substance or mixture may represent.
A.6.2 Classification Criteria for Substances[6]
A.6.2.1 For the purpose of classification for carcinogenicity, substances are allocated to one of two categories based on strength of evidence and additional weight of evidence considerations. In certain instances, route-specific classification may be warranted.
| CATEGORY 1: Known or presumed human carcinogens. The classification of a substance as a Category 1 carcinogen is done on the basis of epidemiological and/or animal data. This classification is further distinguished on the basis of whether the evidence for classification is largely from human data (Category 1A) or from animal data (Category 1B): |
| Category 1A: Known to have carcinogenic potential for humans. Classification in this category is largely based on human evidence. |
| Category 1B: Presumed to have carcinogenic potential for humans. Classification in this category is largely based on animal evidence. The classification of a substance in Category 1A and 1B is based on strength of evidence together with weight of evidence considerations (See paragraph A.6.2.5). Such evidence may be derived from: |
| —human studies that establish a causal relationship between human exposure to a substance and the development of cancer (known human carcinogen); or |
| —animal experiments for which there is sufficient evidence to demonstrate animal carcinogenicity (presumed human carcinogen). |
| In addition, on a case by case basis, scientific judgment may warrant a decision of presumed human carcinogenicity derived from studies showing limited evidence of carcinogenicity in humans together with limited evidence of carcinogenicity in experimental animals. |
| Start Printed Page 9746 |
| CATEGORY 2: Suspected human carcinogens. The classification of a substance in Category 2 is done on the basis of evidence obtained from human and/or animal studies, but which is not sufficiently convincing to place the substance in Category 1A or B. This classification is based on strength of evidence together with weight of evidence considerations (See paragraph A.6.2.5). Such evidence may be from either limited evidence of carcinogenicity in human studies or from limited evidence of carcinogenicity in animal studies. |
| Other considerations: Where the weight of evidence for the carcinogenicity of a substance does not meet the above criteria, any positive study conducted in accordance with established scientific principles, and which reports statistically significant findings regarding the carcinogenic potential of the substance, must be noted on the safety data sheet. |
A.6.2.2 Classification as a carcinogen is made on the basis of evidence from reliable and acceptable methods, and is intended to be used for substances which have an intrinsic property to produce such toxic effects. The evaluations are to be based on all existing data, peer-reviewed published studies and additional data accepted by regulatory agencies.
A.6.2.3Carcinogen classification is a one-step, criterion-based process that involves two interrelated determinations: Evaluations of strength of evidence and consideration of all other relevant information to place substances with human cancer potential into hazard categories.
A.6.2.4Strength of evidence involves the enumeration of tumors in human and animal studies and determination of their level of statistical significance. Sufficient human evidence demonstrates causality between human exposure and the development of cancer, whereas sufficient evidence in animals shows a causal relationship between the agent and an increased incidence of tumors. Limited evidence in humans is demonstrated by a positive association between exposure and cancer, but a causal relationship cannot be stated. Limited evidence in animals is provided when data suggest a carcinogenic effect, but are less than sufficient. (Guidance on consideration of important factors in the classification of carcinogenicity and a more detailed description of the terms "limited" and "sufficient" have been developed by the International Agency for Research on Cancer (IARC) and are provided in non-mandatory appendix F of this section.)
A.6.2.5Weight of evidence: Beyond the determination of the strength of evidence for carcinogenicity, a number of other factors should be considered that influence the overall likelihood that an agent may pose a carcinogenic hazard in humans. The full list of factors that influence this determination is very lengthy, but some of the important ones are considered here.
A.6.2.5.1 These factors can be viewed as either increasing or decreasing the level of concern for human carcinogenicity. The relative emphasis accorded to each factor depends upon the amount and coherence of evidence bearing on each. Generally, there is a requirement for more complete information to decrease than to increase the level of concern. Additional considerations should be used in evaluating the tumor findings and the other factors in a case-by-case manner.
A.6.2.5.2 Some important factors which may be taken into consideration, when assessing the overall level of concern are:
(a) Tumor type and background incidence;
(b) Multisite responses;
(c) Progression of lesions to malignancy;
(d) Reduced tumor latency;
Additional factors which may increase or decrease the level of concern include:
(e) Whether responses are in single or both sexes;
(f) Whether responses are in a single species or several species;
(g) Structural similarity or not to a substance(s) for which there is good evidence of carcinogenicity;
(h) Routes of exposure;
(i) Comparison of absorption, distribution, metabolism and excretion between test animals and humans;
(j) The possibility of a confounding effect of excessive toxicity at test doses; and,
(k) Mode of action and its relevance for humans, such as mutagenicity, cytotoxicity with growth stimulation, mitogenesis, immunosuppression.
Mutagenicity: It is recognized that genetic events are central in the overall process of cancer development. Therefore, evidence of mutagenic activity in vivo may indicate that a substance has a potential for carcinogenic effects.
A.6.2.5.3 A substance that has not been tested for carcinogenicity may in certain instances be classified in Category 1A, Category 1B, or Category 2 based on tumor data from a structural analogue together with substantial support from consideration of other important factors such as formation of common significant metabolites, e.g., for benzidine congener dyes.
A.6.2.5.4 The classification should also take into consideration whether or not the substance is absorbed by a given route(s); or whether there are only local tumors at the site of administration for the tested route(s), and adequate testing by other major route(s) show lack of carcinogenicity.
A.6.2.5.5 It is important that whatever is known of the physico-chemical, toxicokinetic and toxicodynamic properties of the substances, as well as any available relevant information on chemical analogues, i.e., structure activity relationship, is taken into consideration when undertaking classification.
A.6.3 Classification Criteria for Mixtures[7]
A.6.3.1 The mixture shall be classified as a carcinogen when at least one ingredient has been classified as a Category 1 or Category 2 carcinogen and is present at or above the appropriate cut-off value/concentration limit as shown in Table A.6.1.
| Ingredient classified as: | Category 1 carcinogen | Category 2 carcinogen |
|---|---|---|
| Category 1 carcinogen | ≥0.1% | |
| Category 2 carcinogen | ≥0.1% (note 1) | |
| Note: If a Category 2 carcinogen ingredient is present in the mixture at a concentration between 0.1% and 1%, information is required on the SDS for a product. However, a label warning is optional. If a Category 2 carcinogen ingredient is present in the mixture at a concentration of ≥1%, both an SDS and a label is required and the information must be included on each. | ||
Start Printed Page 9747
A.6.3.2 Classification of Mixtures When Data Are Available for the Complete Mixture
A mixture may be classified based on the available test data for the mixture as a whole. In such cases, the test results for the mixture as a whole must be shown to be conclusive taking into account dose and other factors such as duration, observations and analysis (e.g., statistical analysis, test sensitivity) of carcinogenicity test systems.
A.6.3.3 Classification of Mixtures When Data Are Not Available for the Complete Mixture: Bridging Principles
Where the mixture itself has not been tested to determine its carcinogenic hazard, but there are sufficient data on both the individual ingredients and similar tested mixtures to adequately characterize the hazards of the mixture, these data will be used in accordance with the following bridging principles as found in paragraph A.0.5 of this appendix: Dilution; Batching; and Substantially similar mixtures.
A.6.4 Classification of Carcinogenicity[8]
A.6.4.1 Chemical manufacturers, importers and employers evaluating chemicals may treat the following sources as establishing that a substance is a carcinogen or potential carcinogen for hazard communication purposes in lieu of applying the criteria described herein:
A.6.4.1.1 National Toxicology Program (NTP), "Report on Carcinogens" (latest edition);
A.6.4.1.2 International Agency for Research on Cancer (IARC) "Monographs on the Evaluation of Carcinogenic Risks to Humans" (latest editions)
A.6.4.2 Where OSHA has included cancer as a health hazard to be considered by classifiers for a chemical covered by this section subpart, chemical manufacturers, importers, and employers shall classify the chemical as a carcinogen.
A.7 Reproductive Toxicity
A.7.1 Definitions and General Considerations
A.7.1.1Reproductive toxicity refers to adverse effects on sexual function and fertility in adult males and females, as well as developmental toxicity in the offspring, occurring after exposure to a substance or mixture. Some reproductive toxic effects cannot be clearly assigned to either impairment of sexual function and fertility or to developmental toxicity. Nonetheless, substances and mixtures with these effects shall be classified as reproductive toxicants.
For classification purposes, the known induction of genetically based inheritable effects in the offspring is addressed in Germ cell mutagenicity (See A.5).
A.7.1.2Adverse effects on sexual function and fertility means any effect of chemicals that interferes with reproductive ability or sexual capacity. This includes, but is not limited to, alterations to the female and male reproductive system, adverse effects on onset of puberty, gamete production and transport, reproductive cycle normality, sexual behaviour, fertility, parturition, pregnancy outcomes, premature reproductive senescence, or modifications in other functions that are dependent on the integrity of the reproductive systems.
A.7.1.3Adverse effects on development of the offspring means any effect of chemicals which interferes with normal development of the conceptus either before or after birth, which is induced during pregnancy or results from parental exposure. These effects can be manifested at any point in the life span of the organism. The major manifestations of developmental toxicity include death of the developing organism, structural abnormality, altered growth and functional deficiency.
A.7.1.4 Adverse effects on or via lactation are also included in reproductive toxicity, but for classification purposes, such effects are treated separately (See A.7.2.1).
A.7.2 Classification Criteria for Substances
A.7.2.1 For the purpose of classification for reproductive toxicity, substances shall be classified in one of two categories in accordance with Figure A.7.1(a). Effects on sexual function and fertility, and on development, shall be considered. In addition, effects on or via lactation shall be classified in a separate hazard category in accordance with Figure A.7.1(b).
| CATEGORY 1: Known or presumed human reproductive toxicant. Substance shall be classified in Category 1 for reproductive toxicity when they are known to have produced an adverse effect on sexual function and fertility or on development in humans or when there is evidence from animal studies, possibly supplemented with other information, to provide a strong presumption that the substance has the capacity to interfere with reproduction in humans. The classification of a substance is further distinguished on the basis of whether the evidence for classification is primarily from human data (Category 1A) or from animal data (Category 1B). |
| Category 1A: Known human reproductive toxicant. The classification of a substance in this category is largely based on evidence from humans. |
| Category 1B: Presumed human reproductive toxicant. The classification of a substance in this category is largely based on evidence from experimental animals. Data from animal studies shall provide sufficient evidence of an adverse effect on sexual function and fertility or on development in the absence of other toxic effects, or if occurring together with other toxic effects the adverse effect on reproduction is considered not to be a secondary non-specific consequence of other toxic effects. However, when there is mechanistic information that raises doubt about the relevance of the effect for humans, classification in Category 2 may be more appropriate. |
| CATEGORY 2: Suspected human reproductive toxicant. Substances shall be classified in Category 2 for reproductive toxicity when there is some evidence from humans or experimental animals, possibly supplemented with other information, of an adverse effect on sexual function and fertility, or on development, in the absence of other toxic effects, or if occurring together with other toxic effects the adverse effect on reproduction is considered not to be a secondary non-specific consequence of the other toxic effects, and where the evidence is not sufficiently convincing to place the substance in Category 1. For instance, deficiencies in the study may make the quality of evidence less convincing, and in view of this, Category 2 would be the more appropriate classification. |
| Effects on or Via Lactation |
| Effects on or via lactation shall be classified in a separate single category. Chemicals that are absorbed by women and have been shown to interfere with lactation or that may be present (including metabolites) in breast milk in amounts sufficient to cause concern for the health of a breastfed child, shall be classified to indicate this property. Classification for effects via lactation shall be assigned on the basis of: |
| (a) Absorption, metabolism, distribution and excretion studies that indicate the likelihood the substance would be present in potentially toxic levels in breast milk; and/or |
| (b) results of one or two generation studies in animals which provide clear evidence of adverse effect in the offspring due to transfer in the milk or adverse effect on the quality of the milk; and/or |
| (c) human evidence indicating a hazard to babies during the lactation period. |
Start Printed Page 9748
A.7.2.2 Basis of Classification
A.7.2.2.1 Classification is made on the basis of the criteria, outlined above, an assessment of the total weight of evidence, and the use of expert judgment. Classification as a reproductive toxicant is intended to be used for substances which have an intrinsic, specific property to produce an adverse effect on reproduction and substances should not be so classified if such an effect is produced solely as a non-specific secondary consequence of other toxic effects.
A.7.2.2.2 In the evaluation of toxic effects on the developing offspring, it is important to consider the possible influence of maternal toxicity.
A.7.2.2.3 For human evidence to provide the primary basis for a Category 1A classification there must be reliable evidence of an adverse effect on reproduction in humans. Evidence used for classification shall be from well conducted epidemiological studies, if available, which include the use of appropriate controls, balanced assessment, and due consideration of bias or confounding factors. Less rigorous data from studies in humans may be sufficient for a Category 1A classification if supplemented with adequate data from studies in experimental animals, but classification in Category 1B may also be considered.
A.7.2.3 Weight of Evidence
A.7.2.3.1 Classification as a reproductive toxicant is made on the basis of an assessment of the total weight of evidence using expert judgment. This means that all available information that bears on the determination of reproductive toxicity is considered together. Included is information such as epidemiological studies and case reports in humans and specific reproduction studies along with sub-chronic, chronic and special study results in animals that provide relevant information regarding toxicity to reproductive and related endocrine organs. Evaluation of substances chemically related to the material under study may also be included, particularly when information on the material is scarce. The weight given to the available evidence will be influenced by factors such as the quality of the studies, consistency of results, nature and severity of effects, level of statistical significance for intergroup differences, number of endpoints affected, relevance of route of administration to humans and freedom from bias. Both positive and negative results are considered together in a weight of evidence determination. However, a single, positive study performed according to good scientific principles and with statistically or biologically significant positive results may justify classification (See also A.7.2.2.3).
A.7.2.3.2 Toxicokinetic studies in animals and humans, site of action and mechanism or mode of action study results may provide relevant information, which could reduce or increase concerns about the hazard to human health. If it is conclusively demonstrated that the clearly identified mechanism or mode of action has no relevance for humans or when the toxicokinetic differences are so marked that it is certain that the hazardous property will not be expressed in humans then a chemical which produces an adverse effect on reproduction in experimental animals should not be classified.
A.7.2.3.3 In some reproductive toxicity studies in experimental animals the only effects recorded may be considered of low or minimal toxicological significance and classification may not necessarily be the outcome. These effects include, for example, small changes in semen parameters or in the incidence of spontaneous defects in the fetus, small changes in the proportions of common fetal variants such as are observed in skeletal examinations, or in fetal weights, or small differences in postnatal developmental assessments.
A.7.2.3.4 Data from animal studies shall provide sufficient evidence of specific reproductive toxicity in the absence of other systemic toxic effects. However, if developmental toxicity occurs together with other toxic effects in the dam (mother), the potential influence of the generalized adverse effects should be assessed to the extent possible. The preferred approach is to consider adverse effects in the embryo/fetus first, and then evaluate maternal toxicity, along with any other factors which are likely to have influenced these effects, as part of the weight of evidence. In general, developmental effects that are observed at maternally toxic doses should not be automatically discounted. Discounting developmental effects that are observed at maternally toxic doses can only be done on a case-by-case basis when a causal relationship is established or refuted.
A.7.2.3.5 If appropriate information is available it is important to try to determine whether developmental toxicity is due to a specific maternally mediated mechanism or to a non-specific secondary mechanism, like maternal stress and the disruption of homeostasis. Generally, the presence of maternal toxicity should not be used to negate findings of embryo/fetal effects, unless it can be clearly demonstrated that the effects are secondary non-specific effects. This is especially the case when the effects in the offspring are significant, e.g., irreversible effects such as structural malformations. In some situations it is reasonable to assume that reproductive toxicity is due to a secondary consequence of maternal toxicity and discount the effects, for example if the chemical is so toxic that dams fail to thrive and there is severe inanition; they are incapable of nursing pups; or they are prostrate or dying.
A.7.2.4 Maternal Toxicity
A.7.2.4.1 Development of the offspring throughout gestation and during the early postnatal stages can be influenced by toxic effects in the mother either through non-specific mechanisms related to stress and the disruption of maternal homeostasis, or by specific maternally-mediated mechanisms. So, in the interpretation of the developmental outcome to decide classification for developmental effects it is important to consider the possible influence of maternal toxicity. This is a complex issue because of uncertainties surrounding the relationship between maternal toxicity and developmental outcome. Expert judgment and a weight of evidence approach, using all available studies, shall be used to determine the degree of influence to be attributed to maternal toxicity when interpreting the criteria for classification for developmental effects. The adverse effects in the embryo/fetus shall be first considered, and then maternal toxicity, along with any other factors which are likely to have influenced these effects, as weight of evidence, to help reach a conclusion about classification.
A.7.2.4.2 Based on pragmatic observation, it is believed that maternal toxicity may, depending on severity, influence development via non-specific secondary mechanisms, producing effects such as depressed fetal weight, retarded ossification, and possibly resorptions and certain malformations in some strains of certain species. However, the limited numbers of studies which have investigated the relationship between developmental effects and general maternal toxicity have failed to demonstrate a consistent, reproducible relationship across species. Developmental effects which occur even in the presence of maternal toxicity are considered to be evidence of developmental toxicity, unless it can be unequivocally demonstrated on a case by case basis that the developmental effects are secondary to maternal toxicity. Moreover, classification shall be considered where there is a significant toxic effect in the offspring, e.g., irreversible effects such as structural malformations, embryo/fetal lethality, or significant post-natal functional deficiencies.
A.7.2.4.3 Classification shall not automatically be discounted for chemicals that produce developmental toxicity only in association with maternal toxicity, even if a specific maternally-mediated mechanism has been demonstrated. In such a case, classification in Category 2 may be considered more appropriate than Category 1. However, when a chemical is so toxic that maternal death or severe inanition results, or the dams (mothers) are prostrate and incapable of nursing the pups, it is reasonable to assume that developmental toxicity is produced solely as a secondary consequence of maternal toxicity and discount the developmental effects. Classification is not necessarily the outcome in the case of minor developmental changes, e.g., a small reduction in fetal/pup body weight or retardation of ossification when seen in association with maternal toxicity.
A.7.2.4.4 Some of the endpoints used to assess maternal toxicity are provided below. Data on these endpoints, if available, shall be evaluated in light of their statistical or biological significance and dose-response relationship.
(a) Maternal mortality: An increased incidence of mortality among the treated dams over the controls shall be considered evidence of maternal toxicity if the increase occurs in a dose-related manner and can be attributed to the systemic toxicity of the test material. Maternal mortality greater than 10% is considered excessive and the data for that dose level shall not normally be considered to need further evaluation.
(b) Mating index (Number of animals with seminal plugs or sperm/Number of mated × 100). Start Printed Page 9749
(c) Fertility index (Number of animals with implants/Number of matings × 100).
(d) Gestation length (If allowed to deliver).
(e) Body weight and body weight change: Consideration of the maternal body weight change and/or adjusted (corrected) maternal body weight shall be included in the evaluation of maternal toxicity whenever such data are available. The calculation of an adjusted (corrected) mean maternal body weight change, which is the difference between the initial and terminal body weight minus the gravid uterine weight (or alternatively, the sum of the weights of the fetuses), may indicate whether the effect is maternal or intrauterine. In rabbits, the body weight gain may not be a useful indicator of maternal toxicity because of normal fluctuations in body weight during pregnancy.
(f) Food and water consumption (if relevant): The observation of a significant decrease in the average food or water consumption in treated dams (mothers) compared to the control group may be useful in evaluating maternal toxicity, particularly when the test material is administered in the diet or drinking water. Changes in food or water consumption must be evaluated in conjunction with maternal body weights when determining if the effects noted are reflective of maternal toxicity or more simply, unpalatability of the test material in feed or water.
(g) Clinical evaluations (including clinical signs, markers, and hematology and clinical chemistry studies): The observation of increased incidence of significant clinical signs of toxicity in treated dams (mothers) relative to the control group is useful in evaluating maternal toxicity. If this is to be used as the basis for the assessment of maternal toxicity, the types, incidence, degree and duration of clinical signs shall be reported in the study. Clinical signs of maternal intoxication include, but are not limited to: Coma, prostration, hyperactivity, loss of righting reflex, ataxia, or labored breathing.
(h) Post-mortem data: Increased incidence and/or severity of post-mortem findings may be indicative of maternal toxicity. This can include gross or microscopic pathological findings or organ weight data, including absolute organ weight, organ-to-body weight ratio, or organ-to-brain weight ratio. When supported by findings of adverse histopathological effects in the affected organ(s), the observation of a significant change in the average weight of suspected target organ(s) of treated dams (mothers), compared to those in the control group, may be considered evidence of maternal toxicity.
A.7.2.5 Animal and Experimental Data
A.7.2.5.1 A number of scientifically validated test methods are available, including methods for developmental toxicity testing (e.g., OECD Test Guideline 414, ICH Guideline S5A, 1993), methods for peri- and post-natal toxicity testing (e.g., ICH S5B, 1995), and methods for one or two-generation toxicity testing (e.g., OECD Test Guidelines 415, 416, 443).
A.7.2.5.2 Results obtained from screening tests (e.g., OECD Guidelines 421—Reproduction/Developmental Toxicity Screening Test, and 422—Combined Repeated Dose Toxicity Study with Reproduction/Development Toxicity Screening Test) can also be used to justify classification, although the quality of this evidence is less reliable than that obtained through full studies.
A.7.2.5.3 Adverse effects or changes, seen in short- or long-term repeated dose toxicity studies, which are judged likely to impair reproductive function and which occur in the absence of significant generalized toxicity, may be used as a basis for classification, e.g., histopathological changes in the gonads.
A.7.2.5.4 Evidence from in vitro assays, or non-mammalian tests, and from analogous substances using structure-activity relationship (SAR), can contribute to the procedure for classification. In all cases of this nature, expert judgment must be used to assess the adequacy of the data. Inadequate data shall not be used as a primary support for classification.
A.7.2.5.5 It is preferable that animal studies are conducted using appropriate routes of administration which relate to the potential route of human exposure. However, in practice, reproductive toxicity studies are commonly conducted using the oral route, and such studies will normally be suitable for evaluating the hazardous properties of the substance with respect to reproductive toxicity. However, if it can be conclusively demonstrated that the clearly identified mechanism or mode of action has no relevance for humans or when the toxicokinetic differences are so marked that it is certain that the hazardous property will not be expressed in humans then a substance which produces an adverse effect on reproduction in experimental animals should not be classified.
A.7.2.5.6 Studies involving routes of administration such as intravenous or intraperitoneal injection, which may result in exposure of the reproductive organs to unrealistically high levels of the test substance, or elicit local damage to the reproductive organs, e.g., by irritation, must be interpreted with extreme caution and on their own are not normally the basis for classification.
A.7.2.5.7 There is general agreement about the concept of a limit dose, above which the production of an adverse effect may be considered to be outside the criteria which lead to classification. Some test guidelines specify a limit dose, other test guidelines qualify the limit dose with a statement that higher doses may be necessary if anticipated human exposure is sufficiently high that an adequate margin of exposure would not be achieved. Also, due to species differences in toxicokinetics, establishing a specific limit dose may not be adequate for situations where humans are more sensitive than the animal model.
A.7.2.5.8 In principle, adverse effects on reproduction seen only at very high dose levels in animal studies (for example doses that induce prostration, severe inappetence, excessive mortality) do not normally lead to classification, unless other information is available, for example, toxicokinetics information indicating that humans may be more susceptible than animals, to suggest that classification is appropriate.
A.7.2.5.9 However, specification of the actual "limit dose" will depend upon the test method that has been employed to provide the test results.
A.7.3 Classification Criteria for Mixtures[9]
A.7.3.1 Classification of Mixtures When Data Are Available for All Ingredients or Only for Some Ingredients of the Mixture
A.7.3.1.1 The mixture shall be classified as a reproductive toxicant when at least one ingredient has been classified as a Category 1 or Category 2 reproductive toxicant and is present at or above the appropriate cut-off value/concentration limit specified in Table A.7.1 for Category 1 and 2, respectively.
A.7.3.1.2 The mixture shall be classified for effects on or via lactation when at least one ingredient has been classified for effects on or via lactation and is present at or above the appropriate cut-off value/concentration limit specified in Table A.7.1 for the additional category for effects on or via lactation. Start Printed Page 9750
| Ingredient classified as: | Cut-off values/concentration limits triggering classification of a mixture as: | ||
|---|---|---|---|
| Category 1 reproductive toxicant | Category 2 reproductive toxicant | Additional category for effects on or via lactation | |
| Category 1 reproductive toxicant | ≥0.1% | ||
| Category 2 reproductive toxicant | ≥0.1% | ||
| Additional category for effects on or via lactation | ≥0.1% | ||
A.7.3.2 Classification of Mixtures When Data Are Available for the Complete Mixture
Available test data for the mixture as a whole may be used for classification on a case-by-case basis. In such cases, the test results for the mixture as a whole must be shown to be conclusive taking into account dose and other factors such as duration, observations and analysis (e.g., statistical analysis, test sensitivity) of reproduction test systems.
A.7.3.3 Classification of Mixtures When Data Are Not Available for the Complete Mixture: Bridging Principles
A.7.3.3.1 Where the mixture itself has not been tested to determine its reproductive toxicity, but there are sufficient data on both the individual ingredients and similar tested mixtures to adequately characterize the hazards of the mixture, these data shall be used in accordance with the following bridging principles as found in paragraph A.0.5 of this appendix: Dilution, Batching, and Substantially similar mixtures.
A.8 Specific Target Organ Toxicity Single Exposure
A.8.1 Definitions and General Considerations
A.8.1.1Specific target organ toxicity—single exposure, (STOT-SE) refers to specific, non-lethal toxic effects on target organs occurring after a single exposure to a substance or mixture. All significant health effects that can impair function, both reversible and irreversible, immediate and/or delayed and not specifically addressed in A.1 to A.7 and A.10 of this appendix are included. Specific target organ toxicity following repeated exposure is classified in accordance with SPECIFIC TARGET ORGAN TOXICITY—REPEATED EXPOSURE (A.9 of this appendix) and is therefore not included here.
A.8.1.2 Classification identifies the chemical as being a specific target organ toxicant and, as such, it presents a potential for adverse health effects in people who are exposed to it.
A.8.1.3 The adverse health effects produced by a single exposure include consistent and identifiable toxic effects in humans; or, in experimental animals, toxicologically significant changes which have affected the function or morphology of a tissue/organ, or have produced serious changes to the biochemistry or hematology of the organism, and these changes are relevant for human health. Human data is the primary source of evidence for this hazard class.
A.8.1.4 Assessment shall take into consideration not only significant changes in a single organ or biological system but also generalized changes of a less severe nature involving several organs.
A.8.1.5 Specific target organ toxicity can occur by any route that is relevant for humans, i.e., principally oral, dermal or inhalation.
A.8.1.6 The classification criteria for specific target organ toxicity—single exposure are organized as criteria for substances Categories 1 and 2 (See A.8.2.1), criteria for substances Category 3 (See A.8.2.2) and criteria for mixtures (See A.8.3). See also Figure A.8.1.
A.8.2 Classification Criteria for Substances
A.8.2.1 Substances of Category 1 and Category 2
A.8.2.1.1 Substances shall be classified for immediate or delayed effects separately, by the use of expert judgment on the basis of the weight of all evidence available, including the use of recommended guidance values (See A.8.2.1.9). Substances shall then be classified in Category 1 or 2, depending upon the nature and severity of the effect(s) observed, in accordance with Figure A.8.1.
| CATEGORY 1: Substances that have produced significant toxicity in humans, or that, on the basis of evidence from studies in experimental animals can be presumed to have the potential to produce significant toxicity in humans following single exposure: Substances are classified in Category 1 for STOT-SE on the basis of: |
| (a) Reliable and good quality evidence from human cases or epidemiological studies; or |
| (b) observations from appropriate studies in experimental animals in which significant and/or severe toxic effects of relevance to human health were produced at generally low exposure concentrations. Guidance dose/concentration values are provided below (See A.8.2.1.9) to be used as part of weight-of-evidence evaluation. |
| CATEGORY 2: Substances that, on the basis of evidence from studies in experimental animals, can be presumed to have the potential to be harmful to human health following single exposure: Substances are classified in Category 2 for STOT-SE on the basis of observations from appropriate studies in experimental animals in which significant toxic effects, of relevance to human health, were produced at generally moderate exposure concentrations. Guidance dose/concentration values are provided below (See A.8.2.1.9) in order to help in classification. In exceptional cases, human evidence can also be used to place a substance in Category 2 (See A.8.2.1.6). |
| CATEGORY 3: Transient target organ effects: There are target organ effects for which a substance does not meet the criteria to be classified in Categories 1 or 2 indicated above. These are effects which adversely alter human function for a short duration after exposure and from which humans may recover in a reasonable period without leaving significant alteration of structure or function. This category only includes narcotic effects and respiratory tract irritation. Substances are classified specifically for these effects as discussed in A.8.2.2. |
| Note: The primary target organ/system shall be identified where possible, and where this is not possible, the substance shall be identified as a general toxicant. The data shall be evaluated and, where possible, shall not include secondary effects (e.g., a hepatotoxicant can produce secondary effects in the nervous or gastro-intestinal systems). |
A.8.2.1.2 The relevant route(s) of exposure by which the classified substance produces damage shall be identified.
A.8.2.1.3 Classification is determined by expert judgment, on the basis of the weight of all evidence available including the guidance presented below. Start Printed Page 9751
A.8.2.1.4 Weight of evidence of all available data, including human incidents, epidemiology, and studies conducted in experimental animals is used to substantiate specific target organ toxic effects that merit classification.
A.8.2.1.5 The information required to evaluate specific target organ toxicity comes either from single exposure in humans (e.g., exposure at home, in the workplace or environmentally), or from studies conducted in experimental animals. The standard animal studies in rats or mice that provide this information are acute toxicity studies which can include clinical observations and detailed macroscopic and microscopic examination to enable the toxic effects on target tissues/organs to be identified. Results of acute toxicity studies conducted in other species may also provide relevant information.
A.8.2.1.6 In exceptional cases, based on expert judgment, it may be appropriate to place certain substances with human evidence of target organ toxicity in Category 2: (a) When the weight of human evidence is not sufficiently convincing to warrant Category 1 classification, and/or (b) based on the nature and severity of effects. Dose/concentration levels in humans shall not be considered in the classification and any available evidence from animal studies shall be consistent with the Category 2 classification. In other words, if there are also animal data available on the substance that warrant Category 1 classification, the chemical shall be classified as Category 1.
A.8.2.1.7 Effects considered to support classification for Category 1 and 2.
A.8.2.1.7.1 Classification is supported by evidence associating single exposure to the substance with a consistent and identifiable toxic effect.
A.8.2.1.7.2 Evidence from human experience/incidents is usually restricted to reports of adverse health consequences, often with uncertainty about exposure conditions, and may not provide the scientific detail that can be obtained from well-conducted studies in experimental animals.
A.8.2.1.7.3 Evidence from appropriate studies in experimental animals can furnish much more detail, in the form of clinical observations, and macroscopic and microscopic pathological examination and this can often reveal hazards that may not be life-threatening but could indicate functional impairment. Consequently, all available evidence, and relevance to human health, must be taken into consideration in the classification process. Relevant toxic effects in humans and/or animals include, but are not limited to:
(a) Morbidity resulting from single exposure;
(b) Significant functional changes, more than transient in nature, in the respiratory system, central or peripheral nervous systems, other organs or other organ systems, including signs of central nervous system depression and effects on special senses (e.g., sight, hearing and sense of smell);
(c) Any consistent and significant adverse change in clinical biochemistry, hematology, or urinalysis parameters;
(d) Significant organ damage that may be noted at necropsy and/or subsequently seen or confirmed at microscopic examination;
(e) Multi-focal or diffuse necrosis, fibrosis or granuloma formation in vital organs with regenerative capacity;
(f) Morphological changes that are potentially reversible but provide clear evidence of marked organ dysfunction; and,
(g) Evidence of appreciable cell death (including cell degeneration and reduced cell number) in vital organs incapable of regeneration.
A.8.2.1.8 Effects considered not to support classification for Category 1 and 2.
Effects may be seen in humans and/or animals that do not justify classification. Such effects include, but are not limited to:
(a) Clinical observations or small changes in bodyweight gain, food consumption or water intake that may have some toxicological importance but that do not, by themselves, indicate "significant" toxicity;
(b) Small changes in clinical biochemistry, hematology or urinalysis parameters and/or transient effects, when such changes or effects are of doubtful or of minimal toxicological importance;
(c) Changes in organ weights with no evidence of organ dysfunction;
(d) Adaptive responses that are not considered toxicologically relevant; and,
(e) Substance-induced species-specific mechanisms of toxicity, i.e., demonstrated with reasonable certainty to be not relevant for human health, shall not justify classification.
A.8.2.1.9 Guidance values to assist with classification based on the results obtained from studies conducted in experimental animals for Category 1 and 2.
A.8.2.1.9.1 In order to help reach a decision about whether a substance shall be classified or not, and to what degree it shall be classified (Category 1 vs. Category 2), dose/concentration "guidance values" are provided for consideration of the dose/concentration which has been shown to produce significant health effects. The principal argument for proposing such guidance values is that all chemicals are potentially toxic and there has to be a reasonable dose/concentration above which a degree of toxic effect is acknowledged.
A.8.2.1.9.2 Thus, in animal studies, when significant toxic effects are observed that indicate classification, consideration of the dose/concentration at which these effects were seen, in relation to the suggested guidance values, provides useful information to help assess the need to classify (since the toxic effects are a consequence of the hazardous property(ies) and also the dose/concentration).
A.8.2.1.9.3 The guidance value (C) ranges for single-dose exposure which has produced a significant non-lethal toxic effect are those applicable to acute toxicity testing, as indicated in Table A.8.1.
| Guidance value ranges for: | ||||
|---|---|---|---|---|
| Route of exposure | Units | Category 1 | Category 2 | Category 3 |
| Oral (rat) | mg/kg body weight | C ≤ 300 | 2,000 ≥ C > 300 | Guidance values do not apply. |
| Dermal (rat or rabbit) | mg/kg body weight | C ≤ 1,000 | 2,000 ≥ C > 1,000 | |
| Inhalation (rat) gas | ppmV/4h | C ≤ 2,500 | 20,000 ≥ C > 2,500 | |
| Inhalation (rat) vapor | mg/1/4h | C ≤ 10 | 20 ≥ C > 10 | |
| Inhalation (rat) dust/mist/fume | mg/l/4h | C ≤ 1.0 | 5.0 ≥ C > 1.0 | |
A.8.2.1.9.4 The guidance values and ranges mentioned in Table A.8.1 are intended only for guidance purposes, i.e., to be used as part of the weight of evidence approach, and to assist with decisions about classification. They are not intended as strict demarcation values. Guidance values are not provided for Category 3 since this classification is primarily based on human data; animal data may be included in the weight of evidence evaluation.
A.8.2.1.9.5 Thus, it is feasible that a specific profile of toxicity occurs at a dose/concentration below the guidance value, e.g., <2,000 mg/kg body weight by the oral route, however the nature of the effect may result in the decision not to classify. Conversely, a specific profile of toxicity may be seen in animal studies occurring at above a guidance value, e.g., ≥2,000 mg/kg body weight by the oral route, and in addition there is supplementary information from other sources, e.g., other single dose studies, or human case experience, which supports a conclusion that, in view of the weight of evidence, classification is the prudent action to take.
A.8.2.1.10 Other considerations.
A.8.2.1.10.1 When a substance is characterized only by use of animal data the classification process includes reference to dose/concentration guidance values as one of Start Printed Page 9752 the elements that contribute to the weight of evidence approach.
A.8.2.1.10.2 When well-substantiated human data are available showing a specific target organ toxic effect that can be reliably attributed to single exposure to a substance, the substance shall be classified. Positive human data, regardless of probable dose, predominates over animal data. Thus, if a substance is unclassified because specific target organ toxicity observed was considered not relevant or significant to humans, if subsequent human incident data become available showing a specific target organ toxic effect, the substance shall be classified.
A.8.2.1.10.3 A substance that has not been tested for specific target organ toxicity shall, where appropriate, be classified on the basis of data from a scientifically validated structure activity relationship and expert judgment-based extrapolation from a structural analogue that has previously been classified together with substantial support from consideration of other important factors such as formation of common significant metabolites.
A.8.2.2 Substances of Category 3
A.8.2.2.1 Criteria for respiratory tract irritation.
The criteria for classifying substances as Category 3 for respiratory tract irritation are:
(a) Respiratory irritant effects (characterized by localized redness, edema, pruritis and/or pain) that impair function with symptoms such as cough, pain, choking, and breathing difficulties are included. It is recognized that this evaluation is based primarily on human data;
(b) Subjective human observations supported by objective measurements of clear respiratory tract irritation (RTI) (e.g., electrophysiological responses, biomarkers of inflammation in nasal or bronchoalveolar lavage fluids);
(c) The symptoms observed in humans shall also be typical of those that would be produced in the exposed population rather than being an isolated idiosyncratic reaction or response triggered only in individuals with hypersensitive airways. Ambiguous reports simply of "irritation" should be excluded as this term is commonly used to describe a wide range of sensations including those such as smell, unpleasant taste, a tickling sensation, and dryness, which are outside the scope of classification for respiratory tract irritation;
(d) There are currently no scientifically validated animal tests that deal specifically with RTI; however, useful information may be obtained from the single and repeated inhalation toxicity tests. For example, animal studies may provide useful information in terms of clinical signs of toxicity (dyspnoea, rhinitis etc.) and histopathology (e.g., hyperemia, edema, minimal inflammation, thickened mucous layer) which are reversible and may be reflective of the characteristic clinical symptoms described above. Such animal studies can be used as part of weight of evidence evaluation; and,
(e) This special classification will occur only when more severe organ effects including the respiratory system are not observed as those effects would require a higher classification.
A.8.2.2.2 Criteria for narcotic effects.
The criteria for classifying substances in Category 3 for narcotic effects are:
(a) Central nervous system depression including narcotic effects in humans such as drowsiness, narcosis, reduced alertness, loss of reflexes, lack of coordination, and vertigo are included. These effects can also be manifested as severe headache or nausea, and can lead to reduced judgment, dizziness, irritability, fatigue, impaired memory function, deficits in perception and coordination, reaction time, or sleepiness; and,
(b) Narcotic effects observed in animal studies may include lethargy, lack of coordination righting reflex, narcosis, and ataxia. If these effects are not transient in nature, then they shall be considered for classification as Category 1 or 2.
A.8.3 Classification Criteria for Mixtures
A.8.3.1 Mixtures are classified using the same criteria as for substances, or alternatively as described below. As with substances, mixtures may be classified for specific target organ toxicity following single exposure, repeated exposure, or both.
A.8.3.2 Classification of Mixtures When Data Are Available for the Complete Mixture
When reliable and good quality evidence from human experience or appropriate studies in experimental animals, as described in the criteria for substances, is available for the mixture, then the mixture shall be classified by weight of evidence evaluation of this data. Care shall be exercised in evaluating data on mixtures, that the dose, duration, observation or analysis, do not render the results inconclusive.
A.8.3.3 Classification of Mixtures When Data Are Not Available for the Complete Mixture: Bridging Principles
A.8.3.3.1 Where the mixture itself has not been tested to determine its specific target organ toxicity, but there are sufficient data on both the individual ingredients and similar tested mixtures to adequately characterize the hazards of the mixture, these data shall be used in accordance with the following bridging principles as found in paragraph A.0.5 of this appendix: Dilution, Batching, Concentration of mixtures, Interpolation within one hazard category, Substantially similar mixtures, or Aerosols.
A.8.3.4 Classification of Mixtures When Data Are Available for All Ingredients or Only for Some Ingredients of the Mixture
A.8.3.4.1 Where there is no reliable evidence or test data for the specific mixture itself, and the bridging principles cannot be used to enable classification, then classification of the mixture is based on the classification of the ingredient substances. In this case, the mixture shall be classified as a specific target organ toxicant (specific organ specified), following single exposure, repeated exposure, or both when at least one ingredient has been classified as a Category 1 or Category 2 specific target organ toxicant and is present at or above the appropriate cut-off value/concentration limit specified in Table A.8.2 for Categories 1 and 2, respectively.
| Ingredient classified as: | Cut-off values/concentration limits triggering classification of a mixture as: | |
|---|---|---|
| Category 1 | Category 2 | |
| Category 1: Target organ toxicant | ≥1.0% | |
| Category 2: Target organ toxicant | ≥1.0% | |
A.8.3.4.2 These cut-off values and consequent classifications shall be applied equally and appropriately to both single- and repeated-dose target organ toxicants.
A.8.3.4.3 Mixtures shall be classified for either or both single and repeated dose toxicity independently.
A.8.3.4.4 Care shall be exercised when toxicants affecting more than one organ system are combined that the potentiation or synergistic interactions are considered, because certain substances can cause target organ toxicity at <1% concentration when other ingredients in the mixture are known to potentiate its toxic effect.
A.8.3.4.5 Care shall be exercised when extrapolating the toxicity of a mixture that contains Category 3 ingredient(s). A cut-off value/concentration limit of 20%, considered as an additive of all Category 3 ingredients for each hazard endpoint, is appropriate; however, this cut-off value/concentration limit may be higher or lower depending on the Category 3 ingredient(s) involved and the fact that some effects such as respiratory tract irritation may not occur below a certain concentration while other effects such as narcotic effects may occur below this 20% value. Expert judgment shall be exercised. Respiratory tract irritation and narcotic Start Printed Page 9753 effects are to be evaluated separately in accordance with the criteria given in A.8.2.2. When conducting classifications for these hazards, the contribution of each ingredient should be considered additive, unless there is evidence that the effects are not additive.
A.8.3.4.6 In cases where the additivity approach is used for Category 3 ingredients, the "relevant ingredients" of a mixture are those which are present in concentrations ≥1% (w/w for solids, liquids, dusts, mists, and vapours and v/v for gases), unless there is a reason to suspect that an ingredient present at a concentration <1% is still relevant when classifying the mixture for respiratory tract irritation or narcotic effects.
A.9 Specific Target Organ Toxicity Repeated or Prolonged Exposure
A.9.1 Definitions and General Considerations
A.9.1.1Specific target organ toxicity—repeated exposure (STOT-RE) refers to specific toxic effects on target organs occurring after repeated exposure to a substance or mixture. All significant health effects that can impair function, both reversible and irreversible, immediate and/or delayed and not specifically addressed in A.1 to A.7 and A.10 of this appendix are included. Specific target organ toxicity following a single-event exposure is classified in accordance with SPECIFIC TARGET ORGAN TOXICITY-SINGLE EXPOSURE (A.8 of this appendix) and is therefore not included here.
A.9.1.2 Classification identifies the substance or mixture as being a specific target organ toxicant and, as such, it may present a potential for adverse health effects in people who are exposed to it.
A.9.1.3 These adverse health effects produced by repeated exposure include consistent and identifiable toxic effects in humans, or, in experimental animals, toxicologically significant changes which have affected the function or morphology of a tissue/organ, or have produced serious changes to the biochemistry or hematology of the organism and these changes are relevant for human health. Human data will be the primary source of evidence for this hazard class.
A.9.1.4 Assessment shall take into consideration not only significant changes in a single organ or biological system but also generalized changes of a less severe nature involving several organs.
A.9.1.5 Specific target organ toxicity can occur by any route that is relevant for humans, e.g., principally oral, dermal or inhalation.
A.9.2 Classification Criteria for Substances
A.9.2.1 Substances shall be classified as STOT-RE by expert judgment on the basis of the weight of all evidence available, including the use of recommended guidance values which take into account the duration of exposure and the dose/concentration which produced the effect(s), (See A.9.2.9). Substances shall be placed in one of two categories, depending upon the nature and severity of the effect(s) observed, in accordance with Figure A.9.1.
| CATEGORY 1: Substances that have produced significant toxicity in humans, or that, on the basis of evidence from studies in experimental animals can be presumed to have the potential to produce significant toxicity in humans following repeated or prolonged exposure. Substances are classified in Category 1 for specific target organ toxicity (repeated exposure) on the basis of: |
| (a) Reliable and good quality evidence from human cases or epidemiological studies; or, |
| (b) observations from appropriate studies in experimental animals in which significant and/or severe toxic effects, of relevance to human health, were produced at generally low exposure concentrations. Guidance dose/concentration values are provided below (See A.9.2.9) to be used as part of weight-of-evidence evaluation. |
| CATEGORY 2: Substances that, on the basis of evidence from studies in experimental animals can be presumed to have the potential to be harmful to human health following repeated or prolonged exposure. Substances are classified in Category 2 for specific target organ toxicity (repeated exposure) on the basis of observations from appropriate studies in experimental animals in which significant toxic effects, of relevance to human health, were produced at generally moderate exposure concentrations. Guidance dose/concentration values are provided below (See A.9.2.9) in order to help in classification. In exceptional cases human evidence can also be used to place a substance in Category 2 (See A.9.2.6). |
| Note: The primary target organ/system shall be identified where possible, or the substance shall be identified as a general toxicant. The data shall be carefully evaluated and, where possible, shall not include secondary effects (e.g., a hepatotoxicant can produce secondary effects in the nervous or gastro-intestinal systems). |
A.9.2.2 The relevant route of exposure by which the classified substance produces damage shall be identified.
A.9.2.3 Classification is determined by expert judgment, on the basis of the weight of all evidence available including the guidance presented below.
A.9.2.4 Weight of evidence of all data, including human incidents, epidemiology, and studies conducted in experimental animals, is used to substantiate specific target organ toxic effects that merit classification.
A.9.2.5 The information required to evaluate specific target organ toxicity comes either from repeated exposure in humans, e.g., exposure at home, in the workplace or environmentally, or from studies conducted in experimental animals. The standard animal studies in rats or mice that provide this information are 28 day, 90 day or lifetime studies (up to 2 years) that include hematological, clinico-chemical and detailed macroscopic and microscopic examination to enable the toxic effects on target tissues/organs to be identified. Data from repeat dose studies performed in other species may also be used. Other long-term exposure studies, e.g., for carcinogenicity, neurotoxicity or reproductive toxicity, may also provide evidence of specific target organ toxicity that could be used in the assessment of classification.
A.9.2.6 In exceptional cases, based on expert judgment, it may be appropriate to place certain substances with human evidence of specific target organ toxicity in Category 2: (a) When the weight of human evidence is not sufficiently convincing to warrant Category 1 classification, and/or (b) based on the nature and severity of effects. Dose/concentration levels in humans shall not be considered in the classification and any available evidence from animal studies shall be consistent with the Category 2 classification. In other words, if there are also animal data available on the substance that warrant Category 1 classification, the substance shall be classified as Category 1.
A.9.2.7 Effects Considered To Support Classification
A.9.2.7.1 Classification is supported by reliable evidence associating repeated exposure to the substance with a consistent and identifiable toxic effect.
A.9.2.7.2 Evidence from human experience/incidents is usually restricted to reports of adverse health consequences, often with uncertainty about exposure conditions, and may not provide the scientific detail that can be obtained from well-conducted studies in experimental animals.
A.9.2.7.3 Evidence from appropriate studies in experimental animals can furnish much more detail, in the form of clinical observations, hematology, clinical chemistry, macroscopic and microscopic pathological examination and this can often reveal hazards that may not be life-threatening but could indicate functional impairment. Consequently, all available evidence, and relevance to human health, must be taken into consideration in the classification process. Relevant toxic effects in humans and/or animals include, but are not limited to:
(a) Morbidity or death resulting from repeated or long-term exposure. Morbidity or death may result from repeated exposure, even to relatively low doses/concentrations, due to bioaccumulation of the substance or its metabolites, or due to the overwhelming of the de-toxification process by repeated exposure;
(b) Significant functional changes in the central or peripheral nervous systems or other organ systems, including signs of Start Printed Page 9754 central nervous system depression and effects on special senses (e.g., sight, hearing and sense of smell);
(c) Any consistent and significant adverse change in clinical biochemistry, hematology, or urinalysis parameters;
(d) Significant organ damage that may be noted at necropsy and/or subsequently seen or confirmed at microscopic examination;
(e) Multi-focal or diffuse necrosis, fibrosis or granuloma formation in vital organs with regenerative capacity;
(f) Morphological changes that are potentially reversible but provide clear evidence of marked organ dysfunction (e.g., severe fatty change in the liver); and,
(g) Evidence of appreciable cell death (including cell degeneration and reduced cell number) in vital organs incapable of regeneration.
A.9.2.8 Effects Considered Not To Support Classification
Effects may be seen in humans and/or animals that do not justify classification. Such effects include, but are not limited to:
(a) Clinical observations or small changes in bodyweight gain, food consumption or water intake that may have some toxicological importance but that do not, by themselves, indicate "significant" toxicity;
(b) Small changes in clinical biochemistry, hematology or urinalysis parameters and/or transient effects, when such changes or effects are of doubtful or of minimal toxicological importance;
(c) Changes in organ weights with no evidence of organ dysfunction;
(d) Adaptive responses that are not considered toxicologically relevant;
(e) Substance-induced species-specific mechanisms of toxicity, i.e., demonstrated with reasonable certainty to be not relevant for human health, shall not justify classification.
A.9.2.9 Guidance Values To Assist With Classification Based on the Results Obtained From Studies Conducted in Experimental Animals
A.9.2.9.1 In studies conducted in experimental animals, reliance on observation of effects alone, without reference to the duration of experimental exposure and dose/concentration, omits a fundamental concept of toxicology, i.e., all substances are potentially toxic, and what determines the toxicity is a function of the dose/concentration and the duration of exposure. In most studies conducted in experimental animals the test guidelines use an upper limit dose value.
A.9.2.9.2 In order to help reach a decision about whether a substance shall be classified or not, and to what degree it shall be classified (Category 1 vs. Category 2), dose/concentration "guidance values" are provided in Table A.9.1 for consideration of the dose/concentration which has been shown to produce significant health effects. The principal argument for proposing such guidance values is that all chemicals are potentially toxic and there has to be a reasonable dose/concentration above which a degree of toxic effect is acknowledged. Also, repeated-dose studies conducted in experimental animals are designed to produce toxicity at the highest dose used in order to optimize the test objective and so most studies will reveal some toxic effect at least at this highest dose. What is therefore to be decided is not only what effects have been produced, but also at what dose/concentration they were produced and how relevant is that for humans.
A.9.2.9.3 Thus, in animal studies, when significant toxic effects are observed that indicate classification, consideration of the duration of experimental exposure and the dose/concentration at which these effects were seen, in relation to the suggested guidance values, provides useful information to help assess the need to classify (since the toxic effects are a consequence of the hazardous property(ies) and also the duration of exposure and the dose/concentration).
A.9.2.9.4 The decision to classify at all can be influenced by reference to the dose/concentration guidance values at or below which a significant toxic effect has been observed.
A.9.2.9.5 The guidance values refer to effects seen in a standard 90-day toxicity study conducted in rats. They can be used as a basis to extrapolate equivalent guidance values for toxicity studies of greater or lesser duration, using dose/exposure time extrapolation similar to Haber's rule for inhalation, which states essentially that the effective dose is directly proportional to the exposure concentration and the duration of exposure. The assessment should be done on a case-by-case basis; for example, for a 28-day study the guidance values below would be increased by a factor of three.
A.9.2.9.6 Thus for Category 1 classification, significant toxic effects observed in a 90-day repeated-dose study conducted in experimental animals and seen to occur at or below the (suggested) guidance values (C) as indicated in Table A.9.1 would justify classification:
| Route of exposure | Units | Guidance values (dose/concentration) |
|---|---|---|
| Oral (rat) | mg/kg body weight/day | C ≤10 |
| Dermal (rat or rabbit) | mg/kg body weight/day | C ≤20 |
| Inhalation (rat) gas | ppmV/6h/day | C ≤50 |
| Inhalation (rat) vapor | mg/liter/6h/day | C ≤0.2 |
| Inhalation (rat) dust/mist/fume | mg/liter/6h/day | C ≤0.02 |
A.9.2.9.7 For Category 2 classification, significant toxic effects observed in a 90-day repeated-dose study conducted in experimental animals and seen to occur within the (suggested) guidance value ranges as indicated in Table A.9.2 would justify classification:
| Route of exposure | Units | Guidance value range (dose/concentration) |
|---|---|---|
| Oral (rat) | mg/kg body weight/day | 10 <C ≤100 |
| Dermal (rat or rabbit) | mg/kg body weight/day | 20 <C ≤200 |
| Inhalation (rat) gas | ppmV/6h/day | 50 <C ≤250 |
| Inhalation (rat) vapor | mg/liter/6h/day | 0.2 <C ≤1.0 |
| Inhalation (rat) dust/mist/fume | mg/liter/6h/day | 0.02 <C ≤0.2 |
A.9.2.9.8 The guidance values and ranges mentioned in A.2.9.9.6 and A.2.9.9.7 are intended only for guidance purposes, i.e., to be used as part of the weight of evidence approach, and to assist with decisions about Start Printed Page 9755 classification. They are not intended as strict demarcation values.
A.9.2.9.9 Thus, it is possible that a specific profile of toxicity occurs in repeat-dose animal studies at a dose/concentration below the guidance value, e.g., <100 mg/kg body weight/day by the oral route, however the nature of the effect, e.g., nephrotoxicity seen only in male rats of a particular strain known to be susceptible to this effect, may result in the decision not to classify. Conversely, a specific profile of toxicity may be seen in animal studies occurring at above a guidance value, e.g., ≥100 mg/kg body weight/day by the oral route, and in addition there is supplementary information from other sources, e.g., other long-term administration studies, or human case experience, which supports a conclusion that, in view of the weight of evidence, classification is prudent.
A.9.2.10 Other Considerations
A.9.2.10.1 When a substance is characterized only by use of animal data the classification process includes reference to dose/concentration guidance values as one of the elements that contribute to the weight of evidence approach.
A.9.2.10.2 When well-substantiated human data are available showing a specific target organ toxic effect that can be reliably attributed to repeated or prolonged exposure to a substance, the substance shall be classified. Positive human data, regardless of probable dose, predominates over animal data. Thus, if a substance is unclassified because no specific target organ toxicity was seen at or below the dose/concentration guidance value for animal testing, if subsequent human incident data become available showing a specific target organ toxic effect, the substance shall be classified.
A.9.2.10.3 A substance that has not been tested for specific target organ toxicity may in certain instances, where appropriate, be classified on the basis of data from a scientifically validated structure activity relationship and expert judgment-based extrapolation from a structural analogue that has previously been classified together with substantial support from consideration of other important factors such as formation of common significant metabolites.
A.9.3 Classification Criteria for Mixtures
A.9.3.1 Mixtures are classified using the same criteria as for substances, or alternatively as described below. As with substances, mixtures may be classified for specific target organ toxicity following single exposure, repeated exposure, or both.
A.9.3.2 Classification of Mixtures When Data Are Available for the Complete Mixture
When reliable and good quality evidence from human experience or appropriate studies in experimental animals, as described in the criteria for substances, is available for the mixture, then the mixture shall be classified by weight of evidence evaluation of these data. Care shall be exercised in evaluating data on mixtures, that the dose, duration, observation or analysis, do not render the results inconclusive.
A.9.3.3 Classification of Mixtures When Data Are Not Available for the Complete Mixture: Bridging Principles
A.9.3.3.1 Where the mixture itself has not been tested to determine its specific target organ toxicity, but there are sufficient data on both the individual ingredients and similar tested mixtures to adequately characterize the hazards of the mixture, these data shall be used in accordance with the following bridging principles as found in paragraph A.0.5 of this appendix: Dilution; Batching; Concentration of mixtures; Interpolation within one hazard category; Substantially similar mixtures; and Aerosols.
A.9.3.4 Classification of Mixtures When Data Are Available for All Ingredients or Only for Some Ingredients of the Mixture
A.9.3.4.1 Where there is no reliable evidence or test data for the specific mixture itself, and the bridging principles cannot be used to enable classification, then classification of the mixture is based on the classification of the ingredient substances. In this case, the mixture shall be classified as a specific target organ toxicant (specific organ specified), following single exposure, repeated exposure, or both when at least one ingredient has been classified as a Category 1 or Category 2 specific target organ toxicant and is present at or above the appropriate cut-off value/concentration limit specified in Table A.9.3 for Category 1 and 2 respectively.
| Ingredient classified as: | Cut-off values/concentration limits triggering classification of a mixture as: | |
|---|---|---|
| Category 1 | Category 2 | |
| Category 1: Target organ toxicant | ≥1.0% | |
| Category 2: Target organ toxicant | ≥1.0% | |
A.9.3.4.2 These cut-off values and consequent classifications shall be applied equally and appropriately to both single- and repeated-dose target organ toxicants.
A.9.3.4.3 Mixtures shall be classified for either or both single- and repeated-dose toxicity independently.
A.9.3.4.4 Care shall be exercised when toxicants affecting more than one organ system are combined that the potentiation or synergistic interactions are considered, because certain substances can cause specific target organ toxicity at <1% concentration when other ingredients in the mixture are known to potentiate its toxic effect.
A.10 Aspiration Hazard
A.10.1 Definitions and General Considerations
A.10.1.1 Aspiration hazard refers to severe acute effects such as chemical pneumonia, pulmonary injury or death occurring after aspiration of a substance or mixture.
A.10.1.2 Aspiration means the entry of a liquid or solid chemical directly through the oral or nasal cavity, or indirectly from vomiting, into the trachea and lower respiratory system.
A.10.1.3 Aspiration is initiated at the moment of inspiration, in the time required to take one breath, as the causative material lodges at the crossroad of the upper respiratory and digestive tracts in the laryngopharyngeal region.
A.10.1.4 Aspiration of a substance or mixture can occur as it is vomited following ingestion. This may have consequences for labelling, particularly where, due to acute toxicity, a recommendation may be considered to induce vomiting after ingestion. However, if the substance/mixture also presents an aspiration toxicity hazard, the recommendation to induce vomiting may need to be modified.
A.10.1.5 Specific Considerations
A.10.1.5.1 The classification criteria refer to kinematic viscosity. The following provides the conversion between dynamic and kinematic viscosity:

Start Printed Page 9756
A.10.1.5.2 Although the definition of aspiration in A.10.1.1 includes the entry of solids into the respiratory system, classification according to (b) in table A.10.1 for Category 1 is intended to apply to liquid substances and mixtures only.
A.10.1.5.3 Classification of aerosol/mist products
Aerosol and mist products are usually dispensed in containers such as self-pressurized containers, trigger and pump sprayers. Classification for these products shall be considered if their use may form a pool of product in the mouth, which then may be aspirated. If the mist or aerosol from a pressurized container is fine, a pool may not be formed. On the other hand, if a pressurized container dispenses product in a stream, a pool may be formed that may then be aspirated. Usually, the mist produced by trigger and pump sprayers is coarse and therefore, a pool may be formed that then may be aspirated. When the pump mechanism may be removed and contents are available to be swallowed then the classification of the products should be considered.
A.10.2 Classification Criteria for Substances
| Category | Criteria |
|---|---|
| Category 1: Chemicals known to cause human aspiration toxicity hazards or to be regarded as if they cause human aspiration toxicity hazard | A substance shall be classified in Category 1: (a) If reliable and good quality human evidence indicates that it causes aspiration toxicity (See note); or (b) If it is a hydrocarbon and has a kinematic viscosity ≤20.5 mm2/s, measured at 40 °C. |
| Note: Examples of substances included in Category 1 are certain hydrocarbons, turpentine and pine oil. | |
A.10.3 Classification Criteria for Mixtures
A.10.3.1 Classification When Data Are Available for the Complete Mixture
A mixture shall be classified in Category 1 based on reliable and good quality human evidence.
A.10.3.2 Classification of Mixtures When Data Are Not Available for the Complete Mixture: Bridging Principles
A.10.3.2.1 Where the mixture itself has not been tested to determine its aspiration toxicity, but there are sufficient data on both the individual ingredients and similar tested mixtures to adequately characterize the hazard of the mixture, these data shall be used in accordance with the following bridging principles as found in paragraph A.0.5 of this appendix: Dilution; Batching; Concentration of mixtures; Interpolation within one hazard category; and Substantially similar mixtures. For application of the dilution bridging principle, the concentration of aspiration toxicants shall not be less than 10%.
A.10.3.3 Classification of Mixtures When Data Are Available for All Ingredients or Only for Some Ingredients of the Mixture
A.10.3.3.1 The "relevant ingredients" of a mixture are those which are present in concentrations ≥1%.
A.10.3.3.2Category 1
A.10.3.3.2.1 A mixture is classified as Category 1 when the sum of the concentrations of Category 1 ingredients is ≥10%, and the mixture has a kinematic viscosity of ≤20.5 mm2/s, measured at 40 °C.
A.10.3.3.2.2 In the case of a mixture which separates into two or more distinct layers, the entire mixture is classified as Category 1 if in any distinct layer the sum of the concentrations of Category 1 ingredients is ≥10%, and it has a kinematic viscosity of ≤20.5 mm2/s, measured at 40 °C.
Appendix B to § 1910.1200—Physical Hazard Criteria (Mandatory)
B.1 Explosives
B.1.1 Definitions and General Considerations
B.1.1.1 An explosive chemical is a solid or liquid chemical which is in itself capable by chemical reaction of producing gas at such a temperature and pressure and at such a speed as to cause damage to the surroundings. Pyrotechnic chemicals are included even when they do not evolve gases.
A pyrotechnic chemical is a chemical designed to produce an effect by heat, light, sound, gas or smoke or a combination of these as the result of non-detonative self-sustaining exothermic chemical reactions.
An explosive item is an item containing one or more explosive chemicals.
A pyrotechnic item is an item containing one or more pyrotechnic chemicals.
An unstable explosive is an explosive which is thermally unstable and/or too sensitive for normal handling, transport, or use.
An intentional explosive is a chemical or item which is manufactured with a view to produce a practical explosive or pyrotechnic effect.
B.1.1.2 The class of explosives comprises:
(a) Explosive chemicals;
(b) Explosive items, except devices containing explosive chemicals in such quantity or of such a character that their inadvertent or accidental ignition or initiation shall not cause any effect external to the device either by projection, fire, smoke, heat or loud noise; and
(c) Chemicals and items not included under (a) and (b) of this section which are manufactured with the view to producing a practical explosive or pyrotechnic effect.
B.1.2 Classification Criteria
Chemicals and items of this class shall be classified as unstable explosives or shall be assigned to one of the following six divisions depending on the type of hazard they present:
(a) Division 1.1—Chemicals and items which have a mass explosion hazard (a mass explosion is one which affects almost the entire quantity present virtually instantaneously);
(b) Division 1.2—Chemicals and items which have a projection hazard but not a mass explosion hazard;
(c) Division 1.3—Chemicals and items which have a fire hazard and either a minor blast hazard or a minor projection hazard or both, but not a mass explosion hazard:
(i) Combustion of which gives rise to considerable radiant heat; or
(ii) Which burn one after another, producing minor blast or projection effects or both;
(d) Division 1.4—Chemicals and items which present no significant hazard: Chemicals and items which present only a small hazard in the event of ignition or initiation. The effects are largely confined to the package and no projection of fragments of appreciable size or range is to be expected. An external fire shall not cause virtually instantaneous explosion of almost the entire contents of the package;
(e) Division 1.5—Very insensitive chemicals which have a mass explosion hazard: Chemicals which have a mass explosion hazard but are so insensitive that there is very little probability of initiation or of transition from burning to detonation under normal conditions;
(f) Division 1.6—Extremely insensitive items which do not have a mass explosion hazard: Items which predominantly contain extremely insensitive detonating chemicals and which demonstrate a negligible probability of accidental initiation or propagation.
B.1.3 Additional Classification Considerations
B.1.3.1 Explosives shall be classified as unstable explosives or shall be assigned to one of the six divisions identified in B.1.2 in accordance with the three-step procedure in Part I of the UN ST/SG/AC.10 (incorporated by reference; See § 1910.6). The first step is to ascertain whether the substance or mixture has explosive effects (Test Series 1). The second step is the acceptance procedure (Test Series 2 to 4) and the third step is the assignment to a hazard division (Test Series Start Printed Page 9757 5 to 7). The assessment whether a candidate for "ammonium nitrate emulsion or suspension or gel, intermediate for blasting explosives (ANE)" is insensitive enough for inclusion as an oxidizing liquid (See B.13) or an oxidizing solid (See B.14) is determined by Test Series 8 tests.
Note 1: Classification of solid chemicals shall be based on tests performed on the chemical as presented. If, for example, for the purposes of supply or transport, the same chemical is to be presented in a physical form different from that which was tested and which is considered likely to materially alter its performance in a classification test, classification must be based on testing of the chemical in the new form.
Note 2: Some explosive chemicals are wetted with water or alcohols, diluted with other substances or dissolved or suspended in water or other liquid substances to suppress or reduce their explosive properties. These chemicals shall be classified as desensitized explosives (see Chapter B.17).
Note 3: Chemicals with a positive result in Test Series 2 in Part I, Section 12, of UN ST/SG/AC.10/30/Rev.6 (UN Recommendations on the Transport of Dangerous Goods, Manual of Tests and Criteria) (incorporated by reference; see § 1910.6) UN ST/SG/AC.10/30/Rev.6 (UN Recommendations on the Transport of Dangerous Goods, Manual of Tests and Criteria) (incorporated by reference; see § 1910.6)), still have explosive properties. The explosive properties of the chemical shall be communicated in Section 2 (Hazard identification) and Section 9 (Physical and chemical properties) of the Safety Data Sheet, as appropriate.
B.1.3.2 Explosive properties are associated with the presence of certain chemical groups in a molecule which can react to produce very rapid increases in temperature or pressure. The screening procedure in B.1.3.1 is aimed at identifying the presence of such reactive groups and the potential for rapid energy release. If the screening procedure identifies the chemical as a potential explosive, the acceptance procedure (See section 10.3 of the UN ST/SG/AC.10 (incorporated by reference; See § 1910.6)) is necessary for classification.
Note: Neither a Series 1 type (a) propagation of detonation test nor a Series 2 type (a) test of sensitivity to detonative shock is necessary if the exothermic decomposition energy of organic materials is less than 800 J/g.
B.1.3.3 If a mixture contains any known explosives, the acceptance procedure is necessary for classification.
B.1.3.4 A chemical is not classified as explosive if:
(a) There are no chemical groups associated with explosive properties present in the molecule. Examples of groups which may indicate explosive properties are given in Table A6.1 in Appendix 6 of the UN ST/SG/AC.10 (incorporated by reference; See § 1910.6); or
(b) The substance contains chemical groups associated with explosive properties which include oxygen and the calculated oxygen balance is less than −200.
The oxygen balance is calculated for the chemical reaction:
Cx Hy Oz + [x + (y/4)−(z/2)] O2 → x. CO2 + (y/2) H2 O
using the formula:
oxygen balance = −1600 [2x + (y/2) −z]/molecular weight; or
(c) The organic substance or a homogenous mixture of organic substances contains chemical groups associated with explosive properties but the exothermic decomposition energy is less than 500 J/g and the onset of exothermic decomposition is below 500 °C (932 °F). The exothermic decomposition energy may be determined using a suitable calorimetric technique; or
(d) For mixtures of inorganic oxidizing substances with organic material(s), the concentration of the inorganic oxidizing substance is:
(i) Less than 15%, by mass, if the oxidizing substance is assigned to Category 1 or 2;
(ii) less than 30%, by mass, if the oxidizing substance is assigned to Category 3.
B.2 Flammable Gases
B.2.1 Definition
Flammable gas means a gas having a flammable range with air at 20 °C (68 °F) and a standard pressure of 101.3 kPa (14.7 psi).
A pyrophoric gas means a flammable gas that is liable to ignite spontaneously in air at a temperature of 54 °C (130 °F) or below.
A chemically unstable gas means a flammable gas that is able to react explosively even in the absence of air or oxygen.
B.2.2 Classification Criteria
B.2.2.1 A flammable gas shall be classified in Category 1A, 1B, or 2 in accordance with Table B.2.1:
Start Printed Page 9758

B.2.3 Additional Classification Considerations
B.2.3.1 Flammability shall be determined by tests or by calculation in accordance with ISO 10156 (Gases and Gas Mixtures—Determination of Fire Potential and Oxidizing Ability for the Selection of Cylinder Valve Outlets; 1996, first edition or 2010, third edition) (incorporated by reference; see § 1910.6) and, if using fundamental burning velocity for Category 1B, use ISO 817:2014 (third edition) (Refrigerants—Designation and safety classification, Annex C: Method of test for burning velocity measurement of flammable gases) (incorporated by reference; see § 1910.6). Where insufficient data are available to use this method, equivalent validated methods may be used.
B.2.3.2 Pyrophoricity shall be determined at 130 °F (54 °C) in accordance with either IEC 60079-20-1, edition 1.0 (2010-01) (Explosive atmospheres—Part 20-1: Material characteristics for gas and vapor classification—Test methods and data) (incorporated by reference; see § 1910.6) or DIN 51794 (2003) (Determining the ignition temperature of petroleum products) (incorporated by reference; see § 1910.6).
B.2.3.3 The classification procedure for pyrophoric gases need not be applied when experience in production or handling shows that the substance does not ignite spontaneously on coming into contact with air at a temperature of 130 °F (54 °C) or below. Flammable gas mixtures which have not been tested for pyrophoricity and which contain more than one percent pyrophoric components shall be classified as a pyrophoric gas. Expert judgement on the properties and physical hazards of pyrophoric gases and their mixtures should be used in assessing the need for classification of flammable gas mixtures containing one percent or less pyrophoric components. In this case, testing need only be considered if expert judgement indicates a need for additional data to support the classification process.
B.2.3.4 Chemical instability shall be determined in accordance with the method described in Part III of the UN ST/SG/AC.10/30/Rev.6 (UN Recommendations on the Transport of Dangerous Goods, Manual of Tests and Criteria) (incorporated by reference; see § 1910.6)]. If the calculations performed in accordance with ISO 10156 (Gases and Gas Mixtures—Determination of Fire Potential and Oxidizing Ability for the Selection of Cylinder Valve Outlets; 1996, first edition or 2010, third edition) (incorporated by reference; see § 1910.6) show that a gas mixture is not flammable, no additional testing is required for determining chemical instability for classification purposes.
B.3 Aerosols
B.3.1 Definition
Aerosol means any non-refillable receptacle containing a gas compressed, liquefied or dissolved under pressure, and fitted with a release device allowing the contents to be ejected as particles in suspension in a gas, or as a foam, paste, powder, liquid or gas.
B.3.2 Classification Criteria
B.3.2.1 Aerosols are classified in one of three categories, depending on their flammable properties and their heat of combustion. Aerosols shall be considered for classification in Categories 1 or 2 if they contain more than 1% components (by mass) which are classified as flammable in accordance with this appendix, i.e.:
Flammable gases (See B.2);
Flammable liquids (See B.6);
Flammable solids (See B.7);
or if their heat of combustion is at least 20 kJ/g.
Note 1: Flammable components do not include pyrophoric, self-heating or water-reactive chemicals. Start Printed Page 9759
Note 2: Aerosols do not fall additionally within the scope of flammable gases, gases under pressure, flammable liquids, or flammable solids. However, depending on their contents, aerosols may fall within the scope of other hazard classes.
B.3.2.2 An aerosol shall be classified in one of the three categories for this class in accordance with Table B.3.1.
| Category | Criteria |
|---|---|
| 1 | Contains ≥85% flammable components and the chemical heat of combustion is ≥30 kJ/g; or (a) For spray aerosols, in the ignition distance test, ignition occurs at a distance ≥75 cm (29.5 in), or (b) For foam aerosols, in the aerosol foam flammability test (i) The flame height is ≥20 cm (7.87 in) and the flame duration ≥2 s; or (ii) The flame height is ≥4 cm (1.57 in) and the flame duration ≥7 s. |
| 2 | Contains >1% flammable components, or the heat of combustion is ≥20 kJ/g; and (a) for spray aerosols, in the ignition distance test, ignition occurs at a distance ≥15 cm (5.9 in), or in the enclosed space ignition test, the (i) Time equivalent is ≤300 s/m3; or (ii) Deflagration density is ≤300 g/m3 (b) For foam aerosols, in the aerosol foam flammability test, the flame height is ≥4 cm and the flame duration is ≥2 s and it does not meet the criteria for Category 1. |
| 3 | The chemical does not meet the criteria for Categories 1 and 2. The chemical contains ≤1% flammable components (by mass) and has a heat of combustion <20 kJ/g. |
Note: Aerosols containing more than 1% flammable components or with a heat of combustion of at least 20 kJ/g, which are not submitted to the flammability classification procedures in this appendix shall be classified as Category 1.
B.3.3 Additional Classification Considerations
B.3.3.1 To classify an aerosol, data on its flammable components, on its chemical heat of combustion and, if applicable, the results of the aerosol foam flammability test (for foam aerosols) and of the ignition distance test and enclosed space test (for spray aerosols) are necessary.
B.3.3.2 The chemical heat of combustion (ΔHc), in kilojoules per gram (kJ/g), is the product of the theoretical heat of combustion (ΔHcomb), and a combustion efficiency, usually less than 1.0 (a typical combustion efficiency is 0.95 or 95%).
For a composite aerosol formulation, the chemical heat of combustion is the summation of the weighted heats of combustion for the individual components, as follows:

where:
ΔHc = chemical heat of combustion (kJ/g);
wi% = mass fraction of component i in the product;
ΔHc(i) = specific heat of combustion (kJ/g) of component i in the product;
The chemical heats of combustion shall be found in literature, calculated or determined by tests (See ASTM D240-02; ISO 13943, Sections 86.1 to 86.3; and NFPA 30B (incorporated by reference; See § 1910.6)).
B.3.3.3 The Ignition Distance Test, Enclosed Space Ignition Test and Aerosol Foam Flammability Test shall be performed in accordance with sub-sections 31.4, 31.5 and 31.6 of the of the UN ST/SG/AC.10 (incorporated by reference; See § 1910.6).
B.4 Oxidizing Gases
B.4.1 Definition
Oxidizing gas means any gas which may, generally by providing oxygen, cause or contribute to the combustion of other material more than air does.
Note: "Gases which cause or contribute to the combustion of other material more than air does" means pure gases or gas mixtures with an oxidizing power greater than 23.5% (as determined by a method specified in ISO 10156 (Gases and Gas Mixtures—Determination of Fire Potential and Oxidizing Ability for the Selection of Cylinder Valve Outlets; 1996, first edition or 2010, third edition) (incorporated by reference; see § 1910.6) or 10156-2:2005 (E) (Gas cylinders—Gases and Gas Mixtures—Part 2: Determination of Oxidizing Ability of Toxic and Corrosive Gases and Gas Mixtures, First Edition) (incorporated by reference; see § 1910.6) or an equivalent testing method).
B.4.2 Classification Criteria
An oxidizing gas shall be classified in a single category for this class in accordance with Table B.4.1:
| Category | Criteria |
|---|---|
| 1 | Any gas which may, generally by providing oxygen, cause or contribute to the combustion of other material more than air does. |
B.4.3 Additional Classification Considerations
Classification shall be in accordance with tests or calculation methods as described in ISO 10156 (Gases and Gas Mixtures—Determination of Fire Potential and Oxidizing Ability for the Selection of Cylinder Valve Outlets; 1996, first edition or 2010, third edition) (incorporated by reference; see § 1910.6) and ISO 10156-2:2005 (E) (Gas cylinders—Gases and Gas Mixtures—Part 2: Determination of Oxidizing Ability of Toxic and Corrosive Gases and Gas Mixtures, First Edition) (incorporated by reference; see § 1910.6).
B.5 Gases Under Pressure
B.5.1 Definition
Gases under pressure are gases which are contained in a receptacle at a pressure of 200 kPa (29 psi) (gauge) or more at 20 °C (68 °F), or which are liquefied or liquefied and refrigerated. Start Printed Page 9760
They comprise compressed gases, liquefied gases, dissolved gases and refrigerated liquefied gases.
B.5.2 Classification Criteria
Gases under pressure shall be classified in one of four groups in accordance with Table B.5.1:
| Group | Criteria |
|---|---|
| Compressed gas | A gas which when under pressure is entirely gaseous at −50 °C (−58 °F), including all gases with a critical temperature1 ≤−50 °C (−58 °F). |
| Liquefied gas | A gas which when under pressure, is partially liquid at temperatures above −50 °C (−58 °F). A distinction is made between: |
| (a) High pressure liquefied gas: A gas with a critical temperature1 between −50 °C (−58 °F) and +65 °C (149 °F); and | |
| (b) Low pressure liquefied gas: A gas with a critical temperature1 above +65 °C (149 °F). | |
| Refrigerated liquefied gas | A gas which is made partially liquid because of its low temperature. |
| Dissolved gas | A gas which when under pressure is dissolved in a liquid phase solvent. |
| 1The critical temperature is the temperature above which a pure gas cannot be liquefied, regardless of the degree of compression. | |
| Note: Aerosols should not be classified as gases under pressure. See appendix B.3 of this section. | |
B.6 Flammable Liquids
B.6.1 Definition
Flammable liquid means a liquid having a flash point of not more than 93 °C (199.4 °F).
Flash point means the minimum temperature at which a liquid gives off vapor in sufficient concentration to form an ignitable mixture with air near the surface of the liquid, as determined by a method identified in Section B.6.3.
B.6.2 Classification Criteria
A flammable liquid shall be classified in one of four categories in accordance with Table B.6.1:
| Category | Criteria |
|---|---|
| 1 | Flash point <23 °C (73.4 °F) and initial boiling point ≤35 °C (95 °F). |
| 2 | Flash point <23 °C (73.4 °F) and initial boiling point >35 °C (95 °F). |
| 3 | Flash point ≥23 °C (73.4 °F) and ≤60 °C (140 °F). |
| 4 | Flash point >60 °C (140 °F) and ≤93 °C (199.4 °F). |
| Note: Aerosols should not be classified as flammable liquids. See appendix B.3 of this section. | |
B.6.3 Additional Classification Considerations
The flash point shall be determined in accordance with ASTM D56-05, ASTM D3278, ASTM D3828, ASTM D93-08 (incorporated by reference; See § 1910.6), any method specified in 29 CFR 1910.106(a)(14), or any other method specified in GHS Revision 7, Chapter 2.6.
The initial boiling point shall be determined in accordance with ASTM D86-07a or ASTM D1078 (incorporated by reference; see § 1910.6).[9]
B.7 Flammable Solids
B.7.1 Definitions
Flammable solid means a solid which is a readily combustible solid, or which may cause or contribute to fire through friction.
Readily combustible solids are powdered, granular, or pasty chemicals which are dangerous if they can be easily ignited by brief contact with an ignition source, such as a burning match, and if the flame spreads rapidly.
B.7.2 Classification Criteria
B.7.2.1 Powdered, granular or pasty chemicals shall be classified as flammable solids when the time of burning of one or more of the test runs, performed in accordance with the test method described in the UN ST/SG/AC.10 (incorporated by reference; See § 1910.6), Part III, sub-section 33.2.1, is less than 45 s or the rate of burning is more than 2.2 mm/s (0.0866 in/s).
B.7.2.2 Powders of metals or metal alloys shall be classified as flammable solids when they can be ignited and the reaction spreads over the whole length of the sample in 10 min or less.
B.7.2.3 Solids which may cause fire through friction shall be classified in this class by analogy with existing entries (e.g., matches) until definitive criteria are established.
B.7.2.4 A flammable solid shall be classified in one of the two categories for this class using Method N.1 as described in Part III, sub-section 33.2.1 of the UN ST/SG/AC.10 (incorporated by reference; See § 1910.6), in accordance with Table B.7.1:
| Category | Criteria |
|---|---|
| 1 | Burning rate test: |
| Chemicals other than metal powders: | |
| (a) Wetted zone does not stop fire; and | |
| (b) Burning time <45 s or burning rate >2.2 mm/s. | |
| Metal powders: Burning time ≤5 min. | |
| 2 | Burning rate test: |
| Start Printed Page 9761 | |
| Chemicals other than metal powders: | |
| (a) Wetted zone stops the fire for at least 4 min; and | |
| (b) Burning time <45 s or burning rate >2.2 mm/s. | |
| Metal powders: Burning time >5 min and ≤10 min. | |
Note 1: Classification of solid chemicals shall be based on tests performed on the chemical as presented. If, for example, for the purposes of supply or transport, the same chemical is to be presented in a physical form different from that which was tested and which is considered likely to materially alter its performance in a classification test, classification must be based on testing of the chemical in the new form.
Note 2: Aerosols should not be classified as flammable solids. See appendix B.3 of this section.
B.8 Self-Reactive Chemicals
B.8.1 Definitions
Self-reactive chemicals are thermally unstable liquid or solid chemicals liable to undergo a strongly exothermic decomposition even without participation of oxygen (air). This definition excludes chemicals classified under this section as explosives, organic peroxides, oxidizing liquids or oxidizing solids.
A self-reactive chemical is regarded as possessing explosive properties when in laboratory testing the formulation is liable to detonate, to deflagrate rapidly or to show a violent effect when heated under confinement.
B.8.2 Classification Criteria
B.8.2.1 A self-reactive chemical shall be considered for classification in this class unless:
(a) It is classified as an explosive according to B.1 of this appendix;
(b) It is classified as an oxidizing liquid or an oxidizing solid according to B.13 or B.14 of this appendix, except that a mixture of oxidizing substances which contains 5% or more of combustible organic substances shall be classified as a self-reactive chemical according to the procedure defined in B.8.2.2;
(c) It is classified as an organic peroxide according to B.15 of this appendix;
(d) Its heat of decomposition is less than 300 J/g; or
(e) Its self-accelerating decomposition temperature (SADT) is greater than 75 °C (167 °F) for a 50 kg (110 lb) package.
B.8.2.2 Mixtures of oxidizing substances, meeting the criteria for classification as oxidizing liquids or oxidizing solids, which contain 5% or more of combustible organic substances and which do not meet the criteria mentioned in B.8.2.1(a), (c), (d) or (e), shall be subjected to the self-reactive chemicals classification procedure in B.8.2.3. Such a mixture showing the properties of a self-reactive chemical type B to F shall be classified as a self-reactive chemical.
B.8.2.3 Self-reactive chemicals shall be classified in one of the seven categories of "types A to G" for this class, according to the following principles:
(a) Any self-reactive chemical which can detonate or deflagrate rapidly, as packaged, will be defined as self-reactive chemical TYPE A;
(b) Any self-reactive chemical possessing explosive properties and which, as packaged, neither detonates nor deflagrates rapidly, but is liable to undergo a thermal explosion in that package will be defined as self-reactive chemical TYPE B;
(c) Any self-reactive chemical possessing explosive properties when the chemical as packaged cannot detonate or deflagrate rapidly or undergo a thermal explosion will be defined as self-reactive chemical TYPE C;
(d) Any self-reactive chemical which in laboratory testing meets the criteria in (d)(i), (ii), or (iii) will be defined as self-reactive chemical TYPE D:
(i) Detonates partially, does not deflagrate rapidly and shows no violent effect when heated under confinement; or
(ii) Does not detonate at all, deflagrates slowly and shows no violent effect when heated under confinement; or
(iii) Does not detonate or deflagrate at all and shows a medium effect when heated under confinement;
(e) Any self-reactive chemical which, in laboratory testing, neither detonates nor deflagrates at all and shows low or no effect when heated under confinement will be defined as self-reactive chemical TYPE E;
(f) Any self-reactive chemical which, in laboratory testing, neither detonates in the cavitated state nor deflagrates at all and shows only a low or no effect when heated under confinement as well as low or no explosive power will be defined as self-reactive chemical TYPE F;
(g) Any self-reactive chemical which, in laboratory testing, neither detonates in the cavitated state nor deflagrates at all and shows no effect when heated under confinement nor any explosive power, provided that it is thermally stable (self-accelerating decomposition temperature is 60 °C (140 °F) to 75 °C (167 °F) for a 50 kg (110 lb) package), and, for liquid mixtures, a diluent having a boiling point greater than or equal to 150 °C (302 °F) is used for desensitization will be defined as self-reactive chemical TYPE G. If the mixture is not thermally stable or a diluent having a boiling point less than 150 °C (302 °F) is used for desensitization, the mixture shall be defined as self-reactive chemical TYPE F.
B.8.3 Additional Classification Considerations
B.8.3.1 For purposes of classification, the properties of self-reactive chemicals shall be determined in accordance with test series A to H as described in Part II of the UN ST/SG/AC.10 (incorporated by reference; See § 1910.6).
B.8.3.2 Self-accelerating decomposition temperature (SADT) shall be determined in accordance with the UN ST/SG/AC.10, Part II, section 28 (incorporated by reference; See § 1910.6).
B.8.3.3 The classification procedures for self-reactive substances and mixtures need not be applied if:
(a) There are no chemical groups present in the molecule associated with explosive or self-reactive properties; examples of such groups are given in Tables A6.1 and A6.2 in the Appendix 6 of the UN ST/SG/AC.10 (incorporated by reference; See § 1910.6); or
(b) For a single organic substance or a homogeneous mixture of organic substances, the estimated SADT is greater than 75 °C (167 °F) or the exothermic decomposition energy is less than 300 J/g. The onset temperature and decomposition energy may be estimated using a suitable calorimetric technique (See 20.3.3.3 in Part II of the UN ST/SG/AC.10 (incorporated by reference; See § 1910.6)).
B.9 Pyrophoric Liquids
B.9.1 Definition
Pyrophoric liquid means a liquid which, even in small quantities, is liable to ignite within five minutes after coming into contact with air.
B.9.2 Classification Criteria
A pyrophoric liquid shall be classified in a single category for this class using test N.3 in Part III, sub-section 33.3.1.5 of the UN ST/SG/AC.10 (incorporated by reference; See § 1910.6), in accordance with Table B.9.1:
| Category | Criteria |
|---|---|
| 1 | The liquid ignites within 5 min when added to an inert carrier and exposed to air, or it ignites or chars a filter paper on contact with air within 5 min. |
Start Printed Page 9762
B.9.3 Additional Classification Considerations
The classification procedure for pyrophoric liquids need not be applied when experience in production or handling shows that the chemical does not ignite spontaneously on coming into contact with air at normal temperatures (i.e., the substance is known to be stable at room temperature for prolonged periods of time (days)).
B.10 Pyrophoric Solids
B.10.1 Definition
Pyrophoric solid means a solid which, even in small quantities, is liable to ignite within five minutes after coming into contact with air.
B.10.2 Classification Criteria
A pyrophoric solid shall be classified in a single category for this class using test N.2 in Part III, sub-section 33.3.1.4 of the UN ST/SG/AC.10 (incorporated by reference; See § 1910.6), in accordance with Table B.10.1:
| Category | Criteria |
|---|---|
| 1 | The solid ignites within 5 min of coming into contact with air. |
Note: Classification of solid chemicals shall be based on tests performed on the chemical as presented. If, for example, for the purposes of supply or transport, the same chemical is to be presented in a physical form different from that which was tested and which is considered likely to materially alter its performance in a classification test, classification must be based on testing of the chemical in the new form.
B.10.3 Additional Classification Considerations
The classification procedure for pyrophoric solids need not be applied when experience in production or handling shows that the chemical does not ignite spontaneously on coming into contact with air at normal temperatures (i.e., the chemical is known to be stable at room temperature for prolonged periods of time (days)).
B.11 SELF-Heating Chemicals
B.11.1 Definition
A self-heating chemical is a solid or liquid chemical, other than a pyrophoric liquid or solid, which, by reaction with air and without energy supply, is liable to self-heat; this chemical differs from a pyrophoric liquid or solid in that it will ignite only when in large amounts (kilograms) and after long periods of time (hours or days).
Note: Self-heating of a substance or mixture is a process where the gradual reaction of that substance or mixture with oxygen (in air) generates heat. If the rate of heat production exceeds the rate of heat loss, then the temperature of the substance or mixture will rise which, after an induction time, may lead to self-ignition and combustion.
B.11.2 Classification Criteria
B.11.2.1 A self-heating chemical shall be classified in one of the two categories for this class if, in tests performed in accordance with test method N.4 in Part III, sub-section 33.3.1.6 of the UN ST/SG/AC.10 (incorporated by reference; See § 1910.6), the result meets the criteria shown in Table B.11.1.
| Category | Criteria |
|---|---|
| 1 | A positive result is obtained in a test using a 25 mm sample cube at 140 °C (284 °F). |
| 2 | A negative result is obtained in a test using a 25 mm cube sample at 140 °C (284 °F), a positive result is obtained in a test using a 100 mm sample cube at 140 °C (284 °F), and: (a) The unit volume of the chemical is more than 3 m3; or (b) A positive result is obtained in a test using a 100 mm cube sample at 120 °C (248 °F) and the unit volume of the chemical is more than 450 liters; or (c) A positive result is obtained in a test using a 100 mm cube sample at 100 °C (212 °F). |
Note: Classification of solid chemicals shall be based on tests performed on the chemical as presented. If, for example, for the purposes of supply or transport, the same chemical is to be presented in a physical form different from that which was tested and which is considered likely to materially alter its performance in a classification test, classification must be based on testing of the chemical in the new form.
B.11.2.2 Chemicals with a temperature of spontaneous combustion higher than 50°C (122 °F) for a volume of 27 m3 shall not be classified as self-heating chemicals.
B.11.2.3 Chemicals with a spontaneous ignition temperature higher than 50 °C (122 °F) for a volume of 450 liters shall not be classified in Category 1 of this class.
B.11.3 Additional Classification Considerations
B.11.3.1 The classification procedure for self-heating chemicals need not be applied if the results of a screening test can be adequately correlated with the classification test and an appropriate safety margin is applied.
B.11.3.2 Examples of screening tests are:
(a) The Grewer Oven test (VDI guideline 2263, part 1, 1990, Test methods for the Determination of the Safety Characteristics of Dusts) with an onset temperature 80 °K above the reference temperature for a volume of 1 l;
(b) The Bulk Powder Screening Test (Gibson, N. Harper, D.J. Rogers, R. Evaluation of the fire and explosion risks in drying powders, Plant Operations Progress, 4 (3), 181-189, 1985) with an onset temperature 60 °K above the reference temperature for a volume of 1 l.
B.12 Chemicals Which, in Contact With Water, Emit Flammable Gases
B.12.1 Definition
Chemicals which, in contact with water, emit flammable gases are solid or liquid chemicals which, by interaction with water, are liable to become spontaneously flammable or to give off flammable gases in dangerous quantities.
B.12.2 Classification Criteria
B.12.2.1 A chemical which, in contact with water, emits flammable gases shall be classified in one of the three categories for this class, using test N.5 in Part III, sub-section 33.4.1.4 of the UN ST/SG/AC.10 (incorporated by reference; See § 1910.6), in accordance with Table B.12.1: Start Printed Page 9763
| Category | Criteria |
|---|---|
| 1 | Any chemical which reacts vigorously with water at ambient temperatures and demonstrates generally a tendency for the gas produced to ignite spontaneously, or which reacts readily with water at ambient temperatures such that the rate of evolution of flammable gas is equal to or greater than 10 liters per kilogram of chemical over any one minute. |
| 2 | Any chemical which reacts readily with water at ambient temperatures such that the maximum rate of evolution of flammable gas is equal to or greater than 20 liters per kilogram of chemical per hour, and which does not meet the criteria for Category 1. |
| 3 | Any chemical which reacts slowly with water at ambient temperatures such that the maximum rate of evolution of flammable gas is greater than 1 liter per kilogram of chemical per hour, and which does not meet the criteria for Categories 1 and 2. |
Note: Classification of solid chemicals shall be based on tests performed on the chemical as presented. If, for example, for the purposes of supply or transport, the same chemical is to be presented in a physical form different from that which was tested and which is considered likely to materially alter its performance in a classification test, classification must be based on testing of the chemical in the new form.
B.12.2.2 A chemical is classified as a chemical which, in contact with water, emits flammable gases if spontaneous ignition takes place in any step of the test procedure.
B.12.3 Additional Classification Considerations
The classification procedure for this class need not be applied if:
(a) The chemical structure of the chemical does not contain metals or metalloids;
(b) Experience in production or handling shows that the chemical does not react with water, (e.g., the chemical is manufactured with water or washed with water); or
(c) The chemical is known to be soluble in water to form a stable mixture.
B.13 Oxidizing Liquids
B.13.1 Definition
Oxidizing liquid means a liquid which, while in itself not necessarily combustible, may, generally by yielding oxygen, cause, or contribute to, the combustion of other material.
B.13.2 Classification Criteria
An oxidizing liquid shall be classified in one of the three categories for this class using test O.2 in Part III, sub-section 34.4.2 of the UN ST/SG/AC.10 (incorporated by reference; See § 1910.6), in accordance with Table B.13.1:
| Category | Criteria |
|---|---|
| 1 | Any chemical which, in the 1:1 mixture, by mass, of chemical and cellulose tested, spontaneously ignites; or the mean pressure rise time of a 1:1 mixture, by mass, of chemical and cellulose is less than that of a 1:1 mixture, by mass, of 50% perchloric acid and cellulose; |
| 2 | Any chemical which, in the 1:1 mixture, by mass, of chemical and cellulose tested, exhibits a mean pressure rise time less than or equal to the mean pressure rise time of a 1:1 mixture, by mass, of 40% aqueous sodium chlorate solution and cellulose; and the criteria for Category 1 are not met; |
| 3 | Any chemical which, in the 1:1 mixture, by mass, of chemical and cellulose tested, exhibits a mean pressure rise time less than or equal to the mean pressure rise time of a 1:1 mixture, by mass, of 65% aqueous nitric acid and cellulose; and the criteria for Categories 1 and 2 are not met. |
B.13.3 Additional Classification Considerations
B.13.3.1 For organic chemicals, the classification procedure for this class shall not be applied if:
(a) The chemical does not contain oxygen, fluorine or chlorine; or
(b) The chemical contains oxygen, fluorine or chlorine and these elements are chemically bonded only to carbon or hydrogen.
B.13.3.2 For inorganic chemicals, the classification procedure for this class shall not be applied if the chemical does not contain oxygen or halogen atoms.
B.13.3.3 In the event of divergence between test results and known experience in the handling and use of chemicals which shows them to be oxidizing, judgments based on known experience shall take precedence over test results.
B.13.3.4 In cases where chemicals generate a pressure rise (too high or too low), caused by chemical reactions not characterizing the oxidizing properties of the chemical, the test described in Part III, sub-section 34.4.2 of the UN ST/SG/AC.10 (incorporated by reference; See § 1910.6) shall be repeated with an inert substance (e.g., diatomite (kieselguhr)) in place of the cellulose in order to clarify the nature of the reaction.
B.14 Oxidizing Solids
B.14.1 Definition
Oxidizing solid means a solid which, while in itself is not necessarily combustible, may, generally by yielding oxygen, cause, or contribute to, the combustion of other material.
B.14.2 Classification Criteria
An oxidizing solid shall be classified in one of the three categories for this class using test O.1 in Part III, sub-section 34.4.1 or test O.3 in Part III, sub-section 34.4.3, of the UN ST/SG/AC.10 (incorporated by reference; See § 1910.6), in accordance with Table B.14.1:
| Category | Criteria using test O.1 | Criteria using test O.3 |
|---|---|---|
| 1 | Any chemical which, in the 4:1 or 1:1 sample-to-cellulose ratio (by mass) tested, exhibits a mean burning time less than the mean burning time of a 3:2 mixture, (by mass), of potassium bromate and cellulose | Any chemical which, in the 4:1 or 1:1 sample-to-cellulose ratio (by mass) tested, exhibits a mean burning rate greater than the mean burning rate of a 3:1 mixture (by mass) of calcium peroxide and cellulose. |
| Start Printed Page 9764 | ||
| 2 | Any chemical which, in the 4:1 or 1:1 sample-to-cellulose ratio (by mass) tested, exhibits a mean burning time equal to or less than the mean burning time of a 2:3 mixture (by mass) of potassium bromate and cellulose and the criteria for Category 1 are not met | Any chemical which, in the 4:1 or 1:1 sample-to-cellulose ratio (by mass) tested, exhibits a mean burning rate equal to or greater than the mean burning rate of a 1:1 mixture (by mass) of calcium peroxide and cellulose and the criteria for Category 1 are not met. |
| 3 | Any chemical which, in the 4:1 or 1:1 sample-to-cellulose ratio (by mass) tested, exhibits a mean burning time equal to or less than the mean burning time of a 3:7 mixture (by mass) of potassium bromate and cellulose and the criteria for Categories 1 and 2 are not met | Any chemical which, in the 4:1 or 1:1 sample-to-cellulose ratio (by mass) tested, exhibits a mean burning rate equal to or greater than the mean burning rate of a 1:2 mixture (by mass) of calcium peroxide and cellulose and the criteria for Categories 1 and 2 are not met. |
Note 1: Some oxidizing solids may present explosion hazards under certain conditions (e.g., when stored in large quantities). For example, some types of ammonium nitrate may give rise to an explosion hazard under extreme conditions and the "Resistance to detonation test" (International Maritime Solid Bulk Cargoes Code, IMO (IMSBC), Appendix 2, Section 5) may be used to assess this hazard. When information indicates that an oxidizing solid may present an explosion hazard, it shall be indicated on the Safety Data Sheet.
Note 2: Classification of solid chemicals shall be based on tests performed on the chemical as presented. If, for example, for the purposes of supply or transport, the same chemical is to be presented in a physical form different from that which was tested and which is considered likely to materially alter its performance in a classification test, classification must be based on testing of the chemical in the new form.
B.14.3 Additional Classification Considerations
B.14.3.1 For organic chemicals, the classification procedure for this class shall not be applied if:
(a) The chemical does not contain oxygen, fluorine or chlorine; or
(b) The chemical contains oxygen, fluorine or chlorine and these elements are chemically bonded only to carbon or hydrogen.
B.14.3.2 For inorganic chemicals, the classification procedure for this class shall not be applied if the chemical does not contain oxygen or halogen atoms.
B.14.3.3 In the event of divergence between test results and known experience in the handling and use of chemicals which shows them to be oxidizing, judgements based on known experience shall take precedence over test results.
B.15 Organic Peroxides
B.15.1 Definition
B.15.1.1 Organic peroxide means a liquid or solid organic chemical which contains the bivalent -0-0- structure and as such is considered a derivative of hydrogen peroxide, where one or both of the hydrogen atoms have been replaced by organic radicals. The term organic peroxide includes organic peroxide mixtures containing at least one organic peroxide. Organic peroxides are thermally unstable chemicals, which may undergo exothermic self-accelerating decomposition. In addition, they may have one or more of the following properties:
(a) Be liable to explosive decomposition;
(b) Burn rapidly;
(c) Be sensitive to impact or friction;
(d) React dangerously with other substances.
B.15.1.2 An organic peroxide is regarded as possessing explosive properties when in laboratory testing the formulation is liable to detonate, to deflagrate rapidly or to show a violent effect when heated under confinement.
B.15.2 Classification Criteria
B.15.2.1 Any organic peroxide shall be considered for classification in this class, unless it contains:
(a) Not more than 1.0% available oxygen from the organic peroxides when containing not more than 1.0% hydrogen peroxide; or
(b) Not more than 0.5% available oxygen from the organic peroxides when containing more than 1.0% but not more than 7.0% hydrogen peroxide.
Note: The available oxygen content (%) of an organic peroxide mixture is given by the formula:

where:
ni = number of peroxygen groups per molecule of organic peroxide i;
ci = concentration (mass %) of organic peroxide i;
mi = molecular mass of organic peroxide i.
B.15.2.2 Organic peroxides shall be classified in one of the seven categories of "Types A to G" for this class, according to the following principles:
(a) Any organic peroxide which, as packaged, can detonate or deflagrate rapidly shall be defined as organic peroxide TYPE A;
(b) Any organic peroxide possessing explosive properties and which, as packaged, neither detonates nor deflagrates rapidly, but is liable to undergo a thermal explosion in that package shall be defined as organic peroxide TYPE B;
(c) Any organic peroxide possessing explosive properties when the chemical as packaged cannot detonate or deflagrate rapidly or undergo a thermal explosion shall be defined as organic peroxide TYPE C;
(d) Any organic peroxide which in laboratory testing meets the criteria in (d)(i), (ii), or (iii) shall be defined as organic peroxide TYPE D:
(i) Detonates partially, does not deflagrate rapidly and shows no violent effect when heated under confinement; or
(ii) Does not detonate at all, deflagrates slowly and shows no violent effect when heated under confinement; or
(iii) Does not detonate or deflagrate at all and shows a medium effect when heated under confinement;
(e) Any organic peroxide which, in laboratory testing, neither detonates nor deflagrates at all and shows low or no effect when heated under confinement shall be defined as organic peroxide TYPE E;
(f) Any organic peroxide which, in laboratory testing, neither detonates in the cavitated state nor deflagrates at all and shows only a low or no effect when heated under confinement as well as low or no explosive power shall be defined as organic peroxide TYPE F;
(g) Any organic peroxide which, in laboratory testing, neither detonates in the cavitated state nor deflagrates at all and shows no effect when heated under confinement nor any explosive power, provided that it is thermally stable (self-accelerating decomposition temperature is 60 °C (140 °F) or higher for a 50 kg (110 lb) package), and, for liquid mixtures, a diluent having a boiling point of not less than 150 °C (302 °F) is used for desensitization, shall be defined as organic peroxide TYPE G. If the organic peroxide is not thermally stable or a diluent having a boiling point less than 150 °C (302 °F) is used for desensitization, it shall be defined as organic peroxide TYPE F.
B.15.3 Additional Classification Considerations
B.15.3.1 For purposes of classification, the properties of organic peroxides shall be determined in accordance with test series A to H as described in Part II of the UN ST/SG/AC.10 (incorporated by reference; See § 1910.6).
B.15.3.2 Self-accelerating decomposition temperature (SADT) shall be determined in accordance with the UN ST/SG/AC.10 (incorporated by reference; See § 1910.6), Part II, section 28.
B.15.3.3 Mixtures of organic peroxides may be classified as the same type of organic peroxide as that of the most dangerous ingredient. However, as two stable ingredients can form a thermally less stable mixture, the SADT of the mixture shall be determined. Start Printed Page 9765
B.16 Corrosive to Metals
B.16.1 Definition
A chemical which is corrosive to metals means a chemical which by chemical action will materially damage, or even destroy, metals.
B.16.2 Classification criteria
A chemical which is corrosive to metals shall be classified in a single category for this class, using the test in Part III, sub-section 37.4 of the UN ST/SG/AC.10 (incorporated by reference; See § 1910.6), in accordance with Table B.16.1:
| Category | Criteria |
|---|---|
| 1 | Corrosion rate on either steel or aluminium surfaces exceeding 6.25 mm per year at a test temperature of 55 °C (131 °F) when tested on both materials. |
Note: Where an initial test on either steel or aluminium indicates the chemical being tested is corrosive the follow-up test on the other metal is not necessary.
B.16.3 Additional Classification Considerations
The specimen to be used for the test shall be made of the following materials:
(a) For the purposes of testing steel, steel types S235JR+CR (1.0037 resp.St 37-2), S275J2G3+CR (1.0144 resp.St 44-3), ISO 3574, Unified Numbering System (UNS) G 10200, or SAE 1020;
(b) For the purposes of testing aluminium: non-clad types 7075-T6 or AZ5GU-T6.
Chapter B.17
Desensitized Explosives
B.17.1 Definitions and General Considerations
Desensitized explosives are solid or liquid explosive chemicals which are phlegmatized[10] to suppress their explosive properties in such a manner that they do not mass explode and do not burn too rapidly and therefore may be exempted from the hazard class "Explosives" (Chapter B.1; see also Note 2 of paragraph B.1.3).[11]
B.17.1.2 The class of desensitized explosives comprises:
(a) Solid desensitized explosives: Explosive substances or mixtures which are wetted with water or alcohols or are diluted with other substances, to form a homogeneous solid mixture to suppress their explosive properties.
Note: This includes desensitization achieved by formation of hydrates of the substances.
(b) Liquid desensitized explosives: Explosive substances or mixtures which are dissolved or suspended in water or other liquid substances, to form a homogeneous liquid mixture to suppress their explosive properties.
B.17.2 Classification Criteria
B.2.17.2.1 Any explosive which is desensitized shall be considered in this class, unless:
(a) It is intended to produce a practical, explosive or pyrotechnic effect; or
(b) It has a mass explosion hazard according to test series 6(a) or 6(b) or its corrected burning rate according to the burning rate test described in part V, subsection 51.4 of UN ST/SG/AC.10/30/Rev.6 (UN Recommendations on the Transport of Dangerous Goods, Manual of Tests and Criteria) (incorporated by reference; see § 1910.6) is greater than 1200 kg/min; or
(c) Its exothermic decomposition energy is less than 300 J/g.
Note 1: Substances or mixtures which meet the criterion (a) or (b) shall be classified as explosives (see Chapter B.1). Substances or mixtures which meet the criterion (c) may fall within the scope of other physical hazard classes.
Note 2: The exothermic decomposition energy may be estimated using a suitable calorimetric technique (see section 20, sub-section 20.3.3.3 in Part II of UN ST/SG/AC.10/30/Rev.6 (UN Recommendations on the Transport of Dangerous Goods, Manual of Tests and Criteria) (incorporated by reference; see § 1910.6).
B.17.2.2 Desensitized explosives shall be classified in one of the four categories of this class depending on the corrected burning rate (Ac) using the test "burning rate test (external fire)" described in Part V, sub-section 51.4 of UN ST/SG/AC.10/30/Rev.6 (UN Recommendations of the Transport of Dangerous Goods, Manual of Tests and Criteria) (incorporated by reference; see § 1910.6), according to Table B.17.1:
| Category | Criteria |
|---|---|
| 1 | Desensitized explosives with a corrected burning rate (AC) equal to or greater than 300 kg/min but not more than 1200 kg/min. |
| 2 | Desensitized explosives with a corrected burning rate (AC) equal to or greater than 140 kg/min but less than 300 kg/min. |
| 3 | Desensitized explosives with a corrected burning rate (AC) equal to or greater than 60 kg/min but less than 140 kg/min. |
| 4 | Desensitized explosives with a corrected burning rate (AC) less than 60 kg/min. |
Note 1: Desensitized explosives shall be prepared so that they remain homogeneous and do not separate during normal storage and handling, particularly if desensitized by wetting. The manufacturer, importer, or distributor shall provide information in Section 10 of the safety data sheet about the shelf-life and instructions on verifying desensitization. Under certain conditions the content of desensitizing agent (e.g., phlegmatizer, wetting agent or treatment) may decrease during supply and use, and thus, the hazard potential of the desensitized explosive may increase. In addition, Sections 5 and/or 8 of the safety data sheet shall include advice on avoiding increased fire, blast or protection hazards when the chemical is not sufficiently desensitized.
Note 2: Explosive properties of desensitized explosives shall be determined using data from Test Series 2 of UN ST/SG/ Start Printed Page 9766 AC.10/30/Rev.6 (UN Recommendations on the Transport of Dangerous Goods, Manual of Tests and Criteria) (incorporated by reference; see § 1910.6) and shall be communicated in the safety data sheet. For testing of liquid desensitized explosives, refer to section 32, sub-section 32.3.2 of UN ST/SG/AC.10/30/Rev.6 (UN Recommendations on the Transport of Dangerous Goods, Manual of Tests and Criteria) (incorporated by reference; see § 1910.6). Testing of solid desensitized explosives is addressed in section 33, sub-section 33.2.3 of UN ST/SG/AC.10/30/Rev.6 (UN Recommendations on the Transport of Dangerous Goods, Manual of Tests and Criteria) (incorporated by reference; see § 1910.6).
Note 3: Desensitized explosives do not fall additionally within the scope of chapters B.1 (explosives), B.6 (flammable liquids) and B.7 (flammable solids).
B.17.3 Additional Classification Considerations
B.17.3.1 The classification procedure for desensitized explosives does not apply if:
(a) The substances or mixtures contain no explosives according to the criteria in Chapter B.1; or
(b) The exothermic decomposition energy is less than 300 J/g.
B.17.3.2 The exothermic decomposition energy shall be determined using the explosive already desensitized (i.e., the homogenous solid or liquids mixture formed by the explosive and the substance(s) used to suppress its explosive properties). The exothermic decomposition energy may be estimated using a suitable calorimetric technique (see Section 20, sub-section 20.3.3.3 in Part II of UN ST/SG/AC.10/30/Rev. 6 (UN Recommendations on the Transport of Dangerous Goods, Manual of Tests and Criteria) (incorporated by reference; see § 1910.6).
Appendix C to § 1910.1200—Allocation of Label Elements (Mandatory)
C.1 The label for each hazardous chemical shall include the product identifier used on the safety data sheet.
C.1.1 The labels on shipped containers shall also include the name, address, and telephone number of the chemical manufacturer, importer, or responsible party.
C.2 The label for each hazardous chemical that is classified shall include the signal word, hazard statement(s), pictogram(s), and precautionary statement(s) specified in C.4 for each hazard class and associated hazard category, except as provided for in C.2.1 through C.2.4.
C.2.1 Precedence of hazard information
C.2.1.1 If the signal word "Danger" is included, the signal word "Warning" shall not appear;
C.2.1.2 If the skull and crossbones pictogram is included, the exclamation mark pictogram shall not appear where it is used for acute toxicity;
C.2.1.3 If the corrosive pictogram is included, the exclamation mark pictogram shall not appear where it is used for skin or eye irritation;
C.2.1.4 If the health hazard pictogram is included for respiratory sensitization, the exclamation mark pictogram shall not appear where it is used for skin sensitization or for skin or eye irritation.
C.2.2 Hazard statement text
C.2.2.1 The text of all applicable hazard statements shall appear on the label, except as otherwise specified. The information in italics shall be included as part of the hazard statement as provided. For example: "Causes damage to organs (state all organs affected) through prolonged or repeated exposure (state route of exposure if no other routes of exposure cause the hazard)". Hazard statements may be combined where appropriate to reduce the information on the label and improve readability, as long as all of the hazards are conveyed as required.
C.2.2.2 If the chemical manufacturer, importer, or responsible party can demonstrate that all or part of the hazard statement is inappropriate to a specific substance or mixture, the corresponding statement may be omitted from the label.
C.2.3 Pictograms
C.2.3.1 Pictograms shall be in the shape of a square set at a point and shall include a black hazard symbol on a white background with a red frame sufficiently wide to be clearly visible. A square red frame set at a point without a hazard symbol is not a pictogram and is not permitted on the label.
C.2.3.2 One of eight standard hazard symbols shall be used in each pictogram. The eight hazard symbols are depicted in Figure C.1. A pictogram using the exclamation mark symbol is presented in Figure C.2, for the purpose of illustration.
Start Printed Page 9767

C.2.3.3 The exclamation mark pictogram is permitted (but not required) for HNOCs as long as the words "Hazard Not Otherwise Classified" or the letters "HNOC" appear below the pictogram.
C.2.3.4 Pictograms may only appear once on a label. If multiple hazards require the use of the same pictogram, it may not appear a second time on the label.
C.2.4 Precautionary statement text
C.2.4.1 There are four types of precautionary statements presented, "prevention," "response," "storage," and "disposal." The core part of the precautionary statement is presented in bold print. This is the text, except as otherwise specified, that shall appear on the label. Where additional information is required, it is indicated in plain text.
C.2.4.2 When a backslash or diagonal mark (/) appears in the precautionary Start Printed Page 9768 statement text, it indicates that a choice has to be made between the separated phrases. In such cases, the chemical manufacturer, importer, or responsible party can choose the most appropriate phrase(s). For example, "Wear protective gloves/protective clothing/eye protection/face protection" could read "wear eye protection".
C.2.4.3 When three full stops (. . .) appear in the precautionary statement text, they indicate that all applicable conditions are not listed. For example, in "Use explosion-proof electrical/ventilating/lighting/. . ./equipment", the use of ". . ." indicates that other equipment may need to be specified. In such cases, the chemical manufacturer, importer, or responsible party can choose the other conditions to be specified.
C.2.4.4 When text in italics is used in a precautionary statement, this indicates specific conditions applying to the use or allocation of the precautionary statement. For example, "Use explosion-proof electrical/ventilating/lighting/. . ./equipment" is only required for flammable solids "if dust clouds can occur". Text in italics is intended to be an explanatory, conditional note and is not intended to appear on the label.
C.2.4.5 Where square brackets ([ ]) appear around text in a precautionary statement, this indicates that the text in square brackets is not appropriate in every case and should be used only in certain circumstances. In these cases, conditions for use explaining when the text should be used are provided. For example, one precautionary statement states: "[In case of inadequate ventilation] wear respiratory protection." This statement is given with the condition for use "—text in square brackets may be used if additional information is provided with the chemical at the point of use that explains what type of ventilation would be adequate for safe use". This means that, if additional information is provided with the chemical explaining what type of ventilation would be adequate for safe use, the text in square brackets should be used and the statement would read: "In case of inadequate ventilation wear respiratory protection." However, if the chemical is supplied without such ventilation information, the text in square brackets should not be used, and the precautionary statement should read: "Wear respiratory protection."
C.2.4.6 Precautionary statements may be combined or consolidated to save label space and improve readability. For example, "Keep away from heat, sparks and open flame," "Store in a well-ventilated place" and "Keep cool" can be combined to read "Keep away from heat, sparks and open flame and store in a cool, well-ventilated place."
C.2.4.7 Precautionary statements may incorporate minor textual variations from the text prescribed in this appendix if these variations assist in communicating safety information (e.g., spelling variations, synonyms or other equivalent terms) and the safety advice is not diluted or compromised. Any variations must be used consistently on the label and the safety data sheet.
C.2.4.8 In most cases, the precautionary statements are independent (e.g., the phrases for explosive hazards do not modify those related to certain health hazards, and products that are classified for both hazard classes shall bear appropriate precautionary statements for both). Where a chemical is classified for a number of hazards, and the precautionary statements are similar, the most stringent shall be included on the label (this will be applicable mainly to preventive measures).
C.2.4.9 If the chemical manufacturer, importer, or responsible party can demonstrate that a precautionary statement is inappropriate to a specific substance or mixture, the precautionary statement may be omitted from the label.
C.2.4.10 Where a substance or mixture is classified for a number of health hazards, this may trigger multiple precautionary statements relating to medical response, e.g., calling a poison center/doctor/. . . and getting medical advice/attention.
In general, the following principles should be applied:
(a) Where the classification of a substance or mixture triggers several different precautionary statements, a system of prioritization should be applied. Usually, the label need only include one precautionary statement reflecting the response at the highest level with the greatest urgency, which should always be combined with at least one route of exposure or symptom "IF" statement.
(b) Routes of exposure, including "IF exposed or concerned," may be combined when triggered with a medical response statement. If the response statement is triggered with three or more routes of exposure, "IF exposed or concerned" may be used. However, relevant "IF" statements describing symptoms must be included in full. If a route of exposure is triggered multiple times, it need only be included once.
(c) This does not apply to "Get medical advice/attention if you feel unwell" or "Get immediate medical advice/attention" when they are combined with an "If" statement and should appear without prioritization.
C.3 Supplementary hazard information
C.3.1 To ensure that non-standardized information does not lead to unnecessarily wide variation or undermine the required information, supplementary information on the label is limited to when it provides further detail and does not contradict or cast doubt on the validity of the standardized hazard information.
C.3.2 Where the chemical manufacturer, importer, or distributor chooses to add supplementary information on the label, the placement of supplemental information shall not impede identification of information required by this section.
C.3.3 Where an ingredient with unknown acute toxicity is used in a mixture at a concentration ≥1%, and the mixture is not classified based on testing of the mixture as a whole, a statement that X% of the mixture consists of ingredient(s) of unknown acute toxicity (oral/dermal/inhalation) is required on the label and safety data sheet.
Start Printed Page 9769

Start Printed Page 9770

Start Printed Page 9771

Start Printed Page 9772

Start Printed Page 9773

Start Printed Page 9774

Start Printed Page 9775

Start Printed Page 9776

Start Printed Page 9777

Start Printed Page 9778

Start Printed Page 9779

Start Printed Page 9780

Start Printed Page 9781

Start Printed Page 9782

Start Printed Page 9783

Start Printed Page 9784

Start Printed Page 9785

Start Printed Page 9786

Start Printed Page 9787

Start Printed Page 9788

Start Printed Page 9789

Start Printed Page 9790

Start Printed Page 9791

Start Printed Page 9792

Start Printed Page 9793

Start Printed Page 9794

Start Printed Page 9795

Start Printed Page 9796

Start Printed Page 9797

Start Printed Page 9798

Start Printed Page 9799

Start Printed Page 9800

Start Printed Page 9801

Start Printed Page 9802

Start Printed Page 9803

Start Printed Page 9804

Start Printed Page 9805

Start Printed Page 9806

Start Printed Page 9807

Start Printed Page 9808

Start Printed Page 9809

Start Printed Page 9810

Start Printed Page 9811

Start Printed Page 9812

Start Printed Page 9813

Start Printed Page 9814

Start Printed Page 9815

Start Printed Page 9816

Start Printed Page 9817

Start Printed Page 9818

Start Printed Page 9819

Start Printed Page 9820

Start Printed Page 9821

Start Printed Page 9822

Start Printed Page 9823

Start Printed Page 9824

Start Printed Page 9825

Start Printed Page 9826

Start Printed Page 9827

Start Printed Page 9828

Start Printed Page 9829

Start Printed Page 9830
Appendix D to § 1910.1200—Safety Data Sheets (Mandatory)
A safety data sheet (SDS) shall include the information specified in Table D.1 under the section number and heading indicated for sections 1-11 and 16. While each section of the SDS must contain all of the specified information, preparers of safety data sheets are not required to present the information in any particular order within each section. If no relevant information is found for any given subheading within a section, the SDS shall clearly indicate that no applicable information is available. Sections 12-15 may be included in the SDS, but are not mandatory.
| Heading | Subheading |
|---|---|
| 1. Identification | (a) Product identifier used on the label; |
| (b) Other means of identification; | |
| (c) Recommended use of the chemical and restrictions on use; | |
| (d) Name, U.S. address, and U.S. telephone number of the chemical manufacturer, importer, or other responsible party; | |
| (e) Emergency phone number. | |
| 2. Hazard(s) identification | (a) Classification of the chemical in accordance with paragraph (d) of § 1910.1200, including any hazards associated with a change in the chemical's physical form under normal conditions of use; |
| (b) Signal word, hazard statement(s), symbol(s) and precautionary statement(s) in accordance with paragraph (f) of § 1910.1200. (Hazard symbols may be provided as graphical reproductions in black and white or the name of the symbol, e.g., flame, skull and crossbones); | |
| (c) Hazards identified under normal conditions of use that result from a chemical reaction (changing the chemical structure of the original substance or mixture); | |
| (d) Describe any hazards not otherwise classified that have been identified during the classification process; | |
| (e) Where an ingredient with unknown acute toxicity is used in a mixture at a concentration ≥1% and the mixture is not classified based on testing of the mixture as a whole, a statement that X% of the mixture consists of ingredient(s) of unknown acute toxicity is required. | |
| 3. Composition/information on ingredients | Except as provided for in paragraph (i) of § 1910.1200 on trade secrets: For Substances |
| (a) Chemical name; | |
| (b) Common name and synonyms; | |
| (c) CAS number and other unique identifiers; | |
| (d) Impurities and stabilizing additives (constituents) which are themselves classified and which contribute to the classification of the substance. For Mixtures | |
| In addition to the information required for substances: | |
| (a) The chemical name, CAS number or other unique identifier, and concentration (exact percentage) or concentration ranges of all ingredients which are classified as health hazards in accordance with paragraph (d) of § 1910.1200 and (1) are present above their cut-off/concentration limits; or (2) present a health risk below the cut-off/concentration limits. | |
| (b) The concentration (exact percentage) shall be specified unless a trade secret claim is made in accordance with paragraph (i) of § 1910.1200, when there is batch-to-batch variability in the production of a mixture, or for a group of substantially similar mixtures (See A.0.5.1.2) with similar chemical composition. In these cases, concentration ranges may be used. | |
| For All Chemicals Where a Trade Secret is Claimed | |
| Where a trade secret is claimed in accordance with paragraph (i) of § 1910.1200, a statement that the specific chemical identity, exact percentage (concentration), or concentration range of composition has been withheld as a trade secret is required. When the concentration or concentration range is withheld as a trade secret, the chemical composition must be provided in accordance with the prescribed concentration ranges in § 1910.1200(i)(1)(iv). | |
| 4. First-aid measures | (a) Description of necessary measures, subdivided according to the different routes of exposure, i.e., inhalation, skin and eye contact, and ingestion; |
| (b) Most important symptoms/effects, acute and delayed. | |
| (c) Indication of immediate medical attention and special treatment needed, if necessary. | |
| 5. Fire-fighting measures | (a) Suitable (and unsuitable) extinguishing media. |
| (b) Specific hazards arising from the chemical (e.g., nature of any hazardous combustion products). | |
| (c) Special protective equipment and precautions for fire-fighters. | |
| 6. Accidental release measures | (a) Personal precautions, protective equipment, and emergency procedures. |
| (b) Methods and materials for containment and cleaning up. | |
| 7. Handling and storage | (a) Precautions for safe handling. |
| (b) Conditions for safe storage, including any incompatibilities. | |
| 8. Exposure controls/personal protection | (a) For all ingredients or constituents listed in Section 3, the OSHA permissible exposure limit (PEL), American Conference of Governmental Industrial Hygienists (ACGIH) Threshold Limit Value (TLV), and any other exposure limit or range used or recommended by the chemical manufacturer, importer, or employer preparing the safety data sheet, where available. |
| (b) Appropriate engineering controls. | |
| (c) Individual protection measures, such as personal protective equipment. | |
| 9. Physical and chemical properties | (a) Physical state. |
| (b) Color. | |
| (c) Odor. | |
| (d) Melting point/freezing point. | |
| (e) Boiling point (or initial boiling point or boiling range). | |
| (f) Flammability. | |
| Start Printed Page 9831 | |
| (g) Lower and upper explosion limit/flammability limit. | |
| (h) Flash point. | |
| (i) Auto-ignition temperature. | |
| (j) Decomposition temperature. | |
| (k) pH. | |
| (l) Kinematic viscosity. | |
| (m) Solubility. | |
| (n) Partition coefficient n-octanol/water (log value). | |
| (o) Vapor pressure. | |
| (p) Density and/or relative density. | |
| (q) Relative vapor density. | |
| (r) Particle characteristics. | |
| 10. Stability and reactivity | (a) Reactivity; |
| (b) Chemical stability; | |
| (c) Possibility of hazardous reactions, including those associated with foreseeable emergencies; | |
| (d) Conditions to avoid (e.g., static discharge, shock, or vibration); | |
| (e) Incompatible materials; | |
| (f) Hazardous decomposition products. | |
| 11. Toxicological information | Description of the various toxicological (health) effects and the available data used to identify those effects, including: |
| (a) Information on the likely routes of exposure (inhalation, ingestion, skin and eye contact); | |
| (b) Symptoms related to the physical, chemical and toxicological characteristics; | |
| (c) Delayed and immediate effects and also chronic effects from short- and long-term exposure; | |
| (d) Numerical measures of toxicity (such as acute toxicity estimates); | |
| (e) Interactive effects; information on interactions should be included if relevant and readily available; | |
| (f) Whether the hazardous chemical is listed in the National Toxicology Program (NTP) Report on Carcinogens (latest edition) or has been found to be a potential carcinogen in the International Agency for Research on Cancer (IARC) Monographs (latest edition), or by OSHA. | |
| (g) When specific chemical data or information is not available, the preparer must indicate if alternative information is used and the method used to derive the information (e.g., where the preparer is using information from a class of chemicals rather than the exact chemical in question and using SAR to derive the toxicological information). | |
| 12. Ecological information (Non-mandatory) | (a) Ecotoxicity (aquatic and terrestrial, where available); |
| (b) Persistence and degradability; | |
| (c) Bioaccumulative potential; | |
| (d) Mobility in soil; | |
| (e) Other adverse effects (such as hazardous to the ozone layer). | |
| 13. Disposal considerations (Non-mandatory) | Description of waste residues and information on their safe handling and methods of disposal, including the disposal of any contaminated packaging. |
| 14. Transport information (Non-mandatory) | (a) UN number; |
| (b) UN proper shipping name; | |
| (c) Transport hazard class(es); | |
| (d) Packing group, if applicable; | |
| (e) Environmental hazards (e.g., Marine pollutant (Yes/No)); | |
| (f) Transport in bulk according to IMO instruments; | |
| (g) Special precautions which a user needs to be aware of, or needs to comply with, in connection with transport or conveyance either within or outside their premises. | |
| 15. Regulatory information (Non-mandatory) | Safety, health and environmental regulations specific for the product in question. |
| 16. Other information, including date of preparation or last revision | The date of preparation of the SDS or the last change to it. |
* * * * *
BILLING CODE 4510-26-P
BILLING CODE 4510-26-C
BILLING CODE 4510-26-P
BILLING CODE 4510-26-C
BILLING CODE 4510-26-P
BILLING CODE 4510-26-C
BILLING CODE 4510-26-P
BILLING CODE 4510-26-C
BILLING CODE 4510-26-P
BILLING CODE 4510-26-C
BILLING CODE 4510-26-P
BILLING CODE 4510-26-C
BILLING CODE 4510-26-P
BILLING CODE 4510-26-C
BILLING CODE 4510-26-P
BILLING CODE 4510-26-C
BILLING CODE 4510-26-P
BILLING CODE 4510-26-C
BILLING CODE 4510-26-P
BILLING CODE 4510-26-C
BILLING CODE 4510-26-P
BILLING CODE 4510-26-C
BILLING CODE 4510-26-P
BILLING CODE 4510-26-C
[FR Doc. 2020-28987 Filed 2-5-21; 8:45 am]
BILLING CODE 4510-26-P
The Hazard Communication Standard Is Found in 29 Cfr
Source: https://www.federalregister.gov/documents/2021/02/16/2020-28987/hazard-communication-standard
0 Response to "The Hazard Communication Standard Is Found in 29 Cfr"
Post a Comment